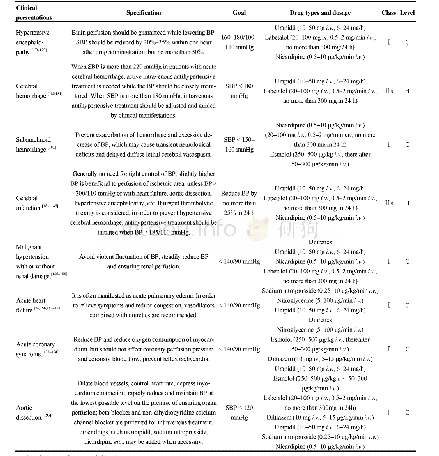
《Table 2 Results of specificity, linearity and LOQ for MAC and its associated impurities》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《指示稳定性的高效液相色谱方法和强制降解研究片剂型马西替坦中的杂质(英文)》
RT:retentio n tim e;RRT:relative retentio n tim e,RT o f im purity peak/RT o f MAC peak;C:m ass co ncentratio ns w ith respect to 5μg/m L limit level,2.2%(LOQ)-215%for Impurity 3,2.1%(LOQ)-209%for Impurity 1,2.6%(LOQ)-264%for Impurity 2,2.5% (L
LOQ is the m inim um am o unt o f analyte that can be quantitatively determ ined w ith precisio n and accuracy using the develo ped m etho d.This can be determ ined by m easuring the signal to no ise(S/N)ratio(w hich sho uld be>10)o f different drug co ncentratio ns and im purity standard so lutio ns.The m inim um co ncentratio ns that can be detected w ith precisio n fo r MAC and its im purities w as established at appro x im ately 0.01%o f 1 000μg/m L w ith an S/N ratio o f≥10 as sho w n in Table 2.
| 图表编号 | XD0041365200 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.08 |
| 作者 | Narasimha S LAKKA、Chandrasekar KUPPAN、Parthasarathy RANGASAMY |
| 绘制单位 | Department of Science and Humanities,VIGNAN's Foundation for Science,Technology & Research (VFSTR)、Department of Analytical Research & Development,Sinotherapeutics Inc.、Department of Science and Humanities,VIGNAN's Foundation for Science,Technology & Re |
| 更多格式 | 高清、无水印(增值服务) |