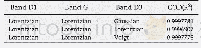《Table 5.Electronic Absorption Spectrum of the Curcumin Calculated by the TDB3LYP/6-31G*Method》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"DFT Study of Physisorption Effect of the Curcumin on CNT(8,0-6) Nanotube for Biological Applications"》
*H-HOMO,L-LUMO
The theoretical absorption spectra of the optimized compounds,such as the molecule curcumin and the complex CNT(8,0-6)/Curcumin,were calculated in the solvent water using the TDB3LYP/6-31G*method.20 excited states were considered for the calculation equations(Table 5).As can be seen from Table 5,the strong absorption peak in the electronic absorption spectrum of the compound Curcumin atλmax=404 nm is observed at oscillator strength f=0.44.This maximum wavelength due to the charge transfer of electron into the excited state S0→S1 is related to three configurations for electron excitations( (H-4→L),(H-2→L) and(H→L)) .The transition from HOMO to LUMO(H→L)is mainly responsible for the formation of the maximum wavelength at 404 nm(Table 5).Fig.8 shows the shape of molecular orbitals participants atλmax=404nm.According to Fig.8 and as it has already been mentioned,most of the charge transfer from HOMO to LUMO in the molecule Curcumin is due to the contribution ofπbonds and lone pairs of the oxygen atoms.The other important excited states are S0→S4,S0→S7 and S0→S13 at 346 nm(f=0.34),315 nm(f=0.32),and 247 nm(f=0.26),respectively.The other excited states of the compound Curcumin have very small intensity(Table 5).The calculated electronic absorption spectrum of the compound Curcumin in the solvent water is observed in Fig.9.
| 图表编号 | XD0034853700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.01 |
| 作者 | SIYAMAK Shahab、MASOOME Sheikhi、MEHRNOOSH Khaleghian、IRYNA Balakhanava、FATEMEH Azarakhshi |
| 绘制单位 | Institute of Physical Organic Chemistry, National Academy of Sciences of Belarus、Institute of Chemistry of New Materials, National Academy of Sciences of Belarus、Belarussian State University、Young Researchers and Elite Club, Gorgan Branch, Islamic Azad Un |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 5.Electronic Absorption Spectrum of the Curcumin Calculated by the TDB3LYP/6-31G*Method”的人还看了
-

- Table 2 Effect of Na Cl stress on the selectivity SK, Na, SCa, Na of ion absorption and transport of A.officinalis seedl