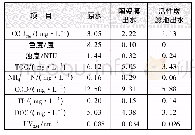
《Tab.1 Antifungal activities of 5-Arylidene-2, 3-diaryl-thiazolidin-4-ones against phytopathogenic f
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"5-芳亚甲基-2,3-二芳基噻唑-4-酮类衍生物的合成及抑菌活性研究(英文)"》
aValues are means of three experiments,standard deviations are given in parentheses;bControl.
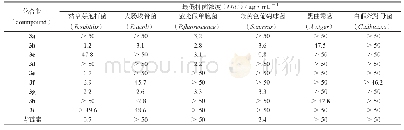
As shown in Tab.1,among all the derivatives,4g and 4n exhibited excellent broad-spectrum antifungal activities against the above-mentioned seven phytopathogenic fungi.For example,the percentage inhibitions of 4g on the growth of Fusariumgraminearum,Alternariaalternata,Fusarium oxysporium f.sp.vasinfectum,Pyricularia oryzae,Valsa mali,Alternaria solani and Alternaria brassicae were 34.94%,40.97%,38.33%,45.04%,43.44%,41.96%,and 47.53%,respectively,and the percentage inhibitions of 4n were 49.26%,56.49%,43.77%,52.76%,46.52%,46.48%and 61.31%,respectively.As shown in the comparative study above,the introduction of heterocyclic groups could generally increase the inhibition rates.On the other hand,some compounds have moderate activity against some phytopathogenic fungi.For example,compounds 4a and4cinhibitedthegrowthofFusarium graminearumby33.23%and30.70%,respectively.Compounds 4a,4c,4e and 4k inhibited the growth of Alternaria solani by 42.28%,37.93%,32.68%and 35.87%,respectively.
| 图表编号 | XD0034395800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.18 |
| 作者 | 於祥、陈娅芳、覃炎、严静 |
| 绘制单位 | 贵州中医药大学贵州省苗医药重点实验室、贵州中医药大学药学院 |
| 更多格式 | 高清、无水印(增值服务) |