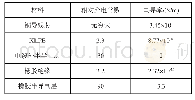
《Table 1–p H, electrical conductivity, alkalinity anions for the supernatants of bauxite residue ame
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
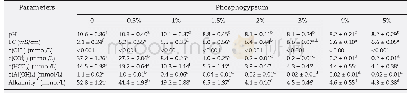
《Phosphogypsum stabilization of bauxite residue:Conversion of its alkaline characteristics》
Different letters of the same line indicate significant differences in the addition of different phosphogypsum(p<0.05).Soluble alkalinity=c(OH-)+c(CO32-)+c(HCO3-)+c(Al (OH)4-) .
The effect of phosphogypsum amendments on the alkalinity,pH,and concentration of soluble anions in the supernatant is presented in Table 1(L/S ratio of 2 mL/g,30°C,24 hr).Due to the existence of a variety of alkaline species(NaOH,Na2CO3,NaHCO3,NaAl (OH)4) ,the pH of the fresh samples was in the range 10 to 11,and the average pH was 10.6.The p H of BR showed a significant reduction with the addition of phosphogypsum,and the final pH of the processed sample was approximately 8.1.Several researchers have examined application of phosphogypsum to alkaline materials(Singh and Garg,2005)and noted pH was reduced after gypsum utilization to BR(Courtney and Timpson,2005;Lee et al.,2007;Lehoux et al.,2013).It has been suggested that an excess of soluble calcium in the form of gypsum can reduce pH to levels suited to plant growth by precipitating soluble alkalinity and suppressing the solubility of solid phase alkalinity(Courtney and Kirwan,2012).The EC was 6.6 mS/cm when phosphogypsum dosage was 3%–5%(0.6–1.0 g),which probably reflected changes in the Na+and Ca2+concentrations.In particular,the phosphogypsum released soluble Ca2+into solution and promoted precipitation of soluble alkaline anions;which caused significant increase in EC.Support for this conclusion was gained from the fact that gypsum amended residue had EC values ranging from 2.1–4.5 mS/cm with higher levels recorded and Ca2+was the major soluble ion in the gypsum amended process(Courtney and Mullen,2009).
| 图表编号 | XD0033514900 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.03.15 |
| 作者 | Shengguo Xue、Meng Li、Jun Jiang、Graeme J.Millar、Chuxuan Li、Xiangfeng Kong |
| 绘制单位 | School of Metallurgy and Environment, Central South University、School of Metallurgy and Environment, Central South University、School of Metallurgy and Environment, Central South University、Institute for Future Environments, Science and Engineering Faculty |
| 更多格式 | 高清、无水印(增值服务) |