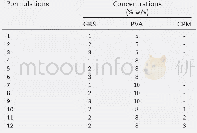
《Table 1.Ion concentration in the degraded solution 10-6》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"Azo dye degradation behavior of AlFeMnTiM(M = Cr, Co, Ni) high-entropy alloys"》
Surface micrographs of SCr,SCo,and SNi after degradation are provided in Fig.8;they demonstrate that nanobristles uniformly and loosely distribute on the surfaces of the three HEA powders.The surface micrographs of the three degraded powders also indicate that the three BM HEA powders exhibit high activity.The nanowhiskers are dense,indicating homogenous corrosion on the surface.As presented in Fig.8(d),the EDS analysis results indicate that these nanobristles are mainly composed of Al,Fe,and O.The p H values of the DB6 solution after being completely degraded by the SCr,SCo and SNi powders are 9.0,8.5,and 8.8,respectively,which means that the solutions are all alkaline after degradation.As evident from Fig.9,the composition of SCr changes somewhat after degradation of DB6.In addition to the FCC and BCC phases,the surface of SCr contains Al2O3,Fe2O3,Cr3O4,Ti2O3,Al OOH,and Fe(OH)3.These phases form because,in the process of azo dye degradation,metals reduce the azo dyes and are oxidized to metal ions,which combine with oxygen in water to generate oxides such as Al2O3,Fe2O3,Cr3O4,and Ti2O3.Metal ions also combine with hydroxyl ions in water to produce hydroxides such as Al OOH and Fe(OH)3.In addition,Table 1 shows that the concentration of each ion remaining in the solution is very low,approaching the lower limit of detection of the instrument.The ICP-AES results indicate that the three HEAs do not cause secondary pollution during the degradation process.
| 图表编号 | XD0028410800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.01 |
| 作者 | Shi-kai Wu、Ye Pan、Ning Wang、Tao Lu、Wei-ji Dai |
| 绘制单位 | School of Materials Science and Engineering, Southeast University, Jiangsu Key Laboratory for Advanced Metallic Materials、School of Materials Science and Engineering, Southeast University, Jiangsu Key Laboratory for Advanced Metallic Materials、School of M |
| 更多格式 | 高清、无水印(增值服务) |