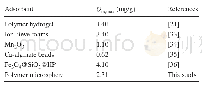
《Table 1–Summary of Congo Red maximum adsorption capacities (qm) on various adsorbents.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《A green method to synthesize flowerlike Fe(OH)_3 microspheres for enhanced adsorption performance toward organic and heavy metal pollutants》
where qeand qt(mg/g)are the normalized amounts of CRadsorbed at equilibrium and at any time t(min),respectively.k1(min-1)and k2(g/ (mg?min)) are the pseudo-first-order and pseudo-second-order rate constants,respectively.The kinetic parameters and the correlation coefficients(R2)can be determined by linear regression(Fig.4c).The experimental value(qe,exp)is 164.90 mg/g.The large differences between the experimental(qe,exp)and calculated values(qe,cal=78.52 mg/g)of the equilibrium adsorption capacity and the relatively low correlation coefficient(R2=0.9651)for the pseudo-first-order model suggests that the adsorption of CR on the Fe(OH)3microspheres does not obey the pseudo-first-order model.Incontrast,the experimental data fit very well to the pseudosecond-order model,with a high correlation coefficient(R2=0.9994).The calculated value(qe,cal=170.07 mg/g)is also very close to qe,exp.The pseudo-second-order model describes very well the adsorption process of CR on the Fe(OH)3microspheres.The adsorption isotherm is shown in Fig.4d.It can be observed that the adsorption capacity qeincreases linearly against initial CR concentration from 0 to 100 mg/L,followed by slower increases from 100 to 1200 mg/L.The highest adsorption capacity of CR by the Fe(OH)3microspheres is about 308 mg/g.Table 1 lists the maximum adsorption capacities(qm)of CR by iron-based adsorbents with different microstructures.Our Fe(OH)3microspheres rank at the top position.We further studied the adsorption mechanism through analyzing the FT-IR spectra of Fe(OH)3before and after adsorption of CR.The results areshown in Fig.3b,where the black,blue and red curves represent the Fe(OH)3before and after adsorption of CR,and CR,respectively.The hydrogen bond peaks of Fe(OH)3at 1480 and1340 cm-1shifted after adsorption of CR,which indicated that the hydrogen bonds were involved in the adsorption behavior.Because there are two NH2groups in the CR molecule,a hydrogen bond between OH from Fe(OH)3and NH2from CRwas formed.
| 图表编号 | XD0025355200 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.11.15 |
| 作者 | Xiaole Zhao、Yingchun Su、Shubin Li、Yajun Bi、Xiaojun Han |
| 绘制单位 | State Key Laboratory of Urban Water Resource and Environment,School of Chemistry and Chemical Engineering,Harbin Institute of Technology、State Key Laboratory of Urban Water Resource and Environment,School of Chemistry and Chemical Engineering,Harbin Insti |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 1–Summary of Congo Red maximum adsorption capacities (qm) on various adsorbents.”的人还看了
-

- 表1 PEI改性磁性四氧化三铁对水中刚果红等温吸附模型参数Tab.1 Isotherm parameters for Congo red adsorption onto surfactant-modified magnetite Fe3O4