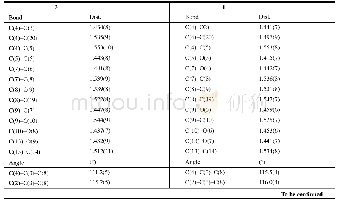
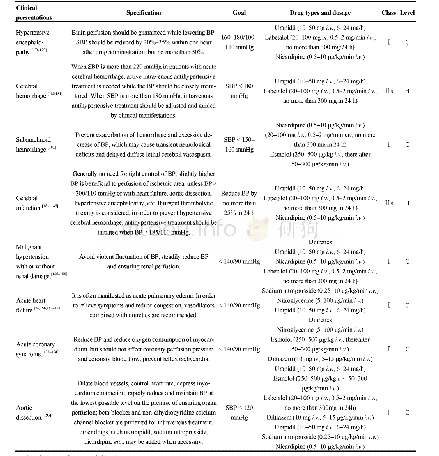
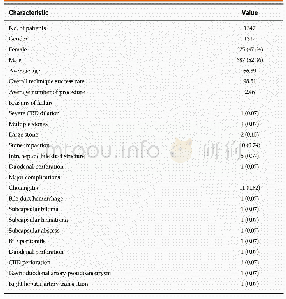
《Table 1Inclusion and exclusion criteria of patient selection.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Novel biomarkers GEP/ABCB5 regulate response to adjuvant transarterial chemoembolization after curative hepatectomy for hepatocellular carcinoma》
TACE:transarterial chemoembolization;HCC:hepatocellular carcinoma;ASA:American Society of Anesthesiologists;ECOG:Eastern Cooperative Oncology Group.
This study was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee and informed consents were obtained from patients.Patients who received curative resection for HCC and fulfilled the inclusion and exclusion criteria in Table 1 were identified from a prospectively collected hepatectomy database.All patients received computed tomography(CT),liver function test and indocyanine green(ICG)clearance test for pre-operative assessment.ICG retention rate at 15 min(ICG R15)were measured by pulse spectrophotometry(LiMON?,Pulsion Medical Systems,Munich,Germany)after injection of an intravenous bolus of 0.25 mg/kg of ICG.Operability was based on radiological resectability and the value of ICG R15.All operations were performed by the same team of surgeons as mentioned previously[7].In short,liver parenchymal transaction was performed with cavitron ultrasonic surgical aspirator(CUSA)and Tissue Link(a radiofrequency saline-linked dissecting sealer)in all cases.Vascular staplers were used to divide major vascular pedicles.Fibrin glue(Tisseel)spray was applied to the parenchymal cut surface of the liver before closure of abdomen.
| 图表编号 | XD0020457500 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.12.15 |
| 作者 | Charinga Ching-Ning Chong、Siu Tim Cheung、Yue-Sun Cheung、Anthony Wing-Hung Chan、Stephen Lam Chan、Simon Chun-Ho Yu、Paul Bo-San Lai |
| 绘制单位 | Department of Surgery, Prince of Wales Hospital, The Chinese University of Hong Kong、Department of Surgery, Prince of Wales Hospital, The Chinese University of Hong Kong、Department of Surgery, Prince of Wales Hospital, The Chinese University of Hong Kong、 |
| 更多格式 | 高清、无水印(增值服务) |