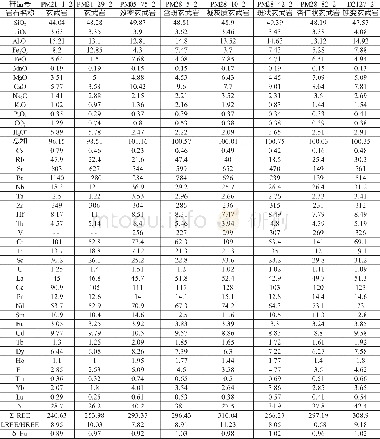
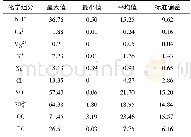
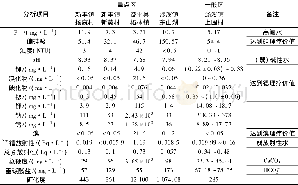
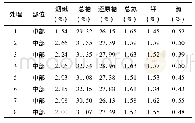
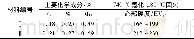《Table 1 Chemical composition of the marked areas in Fig.1》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《pH值对镁合金表面水热沉积磷酸钙涂层微观形貌和腐蚀性能的影响(英文)》
Surface composition of the specimens obtained by EDS is summarized in Table 1.The coatings on all specimens are rich in Ca,P and O.Mg is not detected in the specimen treated at pH=6,while specimens treated at pH=8 and pH=10 contain8.98 at%and 3.16 at%of Mg,respectively,indicating that the coating formed at pH=6 is thicker.In addition to the elements Ca,P and O,a small amount of Na probably from Ca-EDTA solution is detected on the surface of all hydrothermally treated specimens.Fig.2 shows the XRD patterns of the specimens.On the pH=6-treated AZ31,diffraction peaks from OCP(Ca8H2 (PO4)6·5H2O) and a sharp peak at 2θ=53.65°from Mg3(PO4)2 are observed in addition to the peaks from substrate AZ31.When the pH value increases to 8,only the HA(Ca10 (PO4)6(OH)2) peaks are observed.On the pH=10-treated AZ31,the HA peaks are sharper than that of the pH=8 treated specimen,suggesting that the crystallinity of HA on the pH=10-treated specimen is higher.On all the hydrothermally treated specimens,the peak of(002)HA or(002)OCP is higher than that of the other peaks,indicating that the(002)plane of HA or OCP is oriented[21,22].
| 图表编号 | XD0011273800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.10.01 |
| 作者 | 张春艳、张世雨、柳歆鹏、何洪川 |
| 绘制单位 | 重庆理工大学、重庆市特种焊接材料与技术高校工程研究中心、重庆理工大学、重庆理工大学、重庆理工大学 |
| 更多格式 | 高清、无水印(增值服务) |