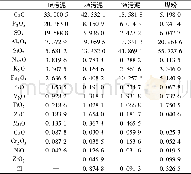
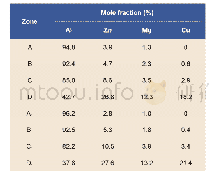
《Table 2 Chemical composition of points 1 and 2 marked in Fig.3 obtained by EDS (at%)》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《ZrO_2(ZrB_2)活性扩散障界面演变行为的研究(英文)》
Further investigation with EDS point scanning analysis of the Zr-rich layer(the interlayer in the active diffusion barrier structure)of the two coating systems(see Table 2)shows that,the Ni,Cr,Al atom contents in the Zr-rich layer of the N5/Zr O2/Ni Cr Al system are higher than those in the Zr-rich layer of the N5/BDSZ/Ni Cr Al system,indicating that more substances with Ni,Cr,Al phases are formed around the Zr-rich layer of the N5/Zr O2/Ni Cr Al system.The formed substances primarily consist of Al2O3,oxygen-deficient zirconia and mixed phases of Ni and Cr.With the increase of high-temperature oxidation time,the presence of Ni and Cr mixed phase will lead to the volume expansion of the interfacial of Al2O3/Zr-rich layer,the increase in stress and eventually the formation of cracks.Therefore,in terms of the N5/Zr O2/Ni Cr Al system,once cracks appear,they will expand rapidly and result in interfacial fracture,since the formed\""sandwich\""active diffusion barrier is primarily composed of ceramic phases.In terms of the N5/BDSZ/Ni Cr Al system,although mixed phase is also formed in the Al2O3/Zr-rich layer/Al2O3\""sandwich\""structure,the presence of Zr B2 will play the role of dispersion strengthening,which can increase the toughness of such a\""sandwich\""structure,and prevent the generation and expansion of cracks,thereby increasing the high-temperature service life of the active diffusion barrier structure.
| 图表编号 | XD0011237900 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.05.01 |
| 作者 | 刘林涛、李争显、杨晨曦、何飞 |
| 绘制单位 | 西安建筑科技大学、西安建筑科技大学、西北有色金属研究院、西安建筑科技大学、西北有色金属研究院 |
| 更多格式 | 高清、无水印(增值服务) |