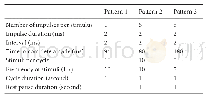
《Table 1IC50values of chrysin derivatives towards two selected tumor cell lines.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Synthesis and anti-tumor activities of novel 7-O-amino acids chrysin derivatives》
IC50:concentration of compound that is required for 50%inhibition of MGC-803 or MCF-7 cell lines after a continuous exposure of 48 h,as measured by MTT assay.5-FU was used as positive control.
In vitro antiproliferative activities chrysin 3a–6l were studied on human gastric carcinoma cell lines MGC-803 and human breast carcinoma cell lines MCF-7 by standard MTT assay.The assays were performed in 96-well plates(Mosmann,1983).The IC50values of the compounds against MGC-803 cell lines and MCF-7 cell lines are summarized in Table 1.To our delight,most of these compounds exhibit a dramastic increase in inhibitory potency of MGC-803 cell lines,compared with chrysin.For MGC-803 cell lines,compounds5b,5d,5i,5k,and 6f showed a noteworthy cytotoxic activity.Mean IC50values are higher 2-and 1.5-fold than chrysin and 5-FU,respectively.In general,compounds with better anti-tumor activities are chrysin amino acid esters but do not indicate a clear regular on the selectivity of substituent group.While for MCF-7 cell lines,compounds 5g and 5j possessed extreme anti-tumor activities compared with chrysin,showed stronger inhibition rate than 5-FU in addition to a series of compounds that are stronger antitumor activities than chrysin.In particular,these compounds which contained short carbon chains linking amino acids ester showed excellent antiproliferative activities of MCF-7 cell lines.It is presented strongest antiproliferative activities especially when the substituents are a small group(H,–CH3).According to MTT assay,we speculate that hydrolysis products 6a–6l could present lower tumor inhibitory rate since carboxylate changed the pH of the environment surrounding the cells.On the other hand,too long carbon chain hindered the binding of the drug to the active site at the protein.
| 图表编号 | XD008002700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.07.18 |
| 作者 | Ding Liu、Yan-peng Li、Hong-xiu Shen、Yang Li、Jun He、Qi-zhi Zhang、Yun-mei Liu |
| 绘制单位 | Institute of Pharmacy & Pharmacology,Hunan Province Cooperative Innovation Center for Molecular Target New Drug Study,University of South China、Institute of Pharmacy & Pharmacology,Hunan Province Cooperative Innovation Center for Molecular Target New Drug |
| 更多格式 | 高清、无水印(增值服务) |



![Table 2.comparison of the cost and product values for two processes[57].](http://bookimg.mtoou.info/tubiao/gif/TRQZ201901018_49200.gif)