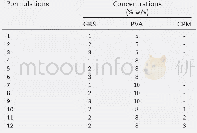
《Table 2 Leaching results of roasted kaolin in alkali solution》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《还原焙烧高岭土中氧化铝和氧化硅的碱浸高效分离(英文)》
Al2O3(T),SiO2(T)and Na2O(T):Total contents of Al2O3,SiO2 and Na2O in residue,respectively;Al2O3(S),SiO2(S):Al2O3 and SiO2 contents of sodalite in residue,respectively;SiO2(Q):Quartz content in residue;(A/S)1=w(Al2O3 (T)) /w(SiO2 (T))
The composition of Al-Si spinel can be calculated by leaching rate of amorphous silica treated with dilute NaOH solution;however,the major problem arises in determining the end-point,particularly of NaOH solution-free amorphous SiO2 reaction in the presence of other reactions[15].Therefore,a method was proposed in this work to deduce the composition of Al-Si spinel by A/S in the leached residue calculated in the following way.The total percentages of Al2O3(T),Si O2(T)and Na2O(T)in residue were firstly analyzed.Based on the amount of Na2O(T),the corresponding amounts ofAl2O3(S)and SiO2(S)in DSP were calculated according to the formula of DSP.Meanwhile,the SiO2(Q)content in residue could also be calculated provided that natural quartz is inactive in NaOH solution at atmospheric pressure.Subsequently the Al2O3 content in Al-Si spinel can be calculated by subtracting Al2O3(S)content from Al2O3(T)content similarly,the SiO2 content in Al-Si spinel can be calculated by subtracting SiO2(S)content and SiO2(Q)content from SiO2(T)content.Therefore,the A/S ratio in Al-Si spinel is obtained.In order to decrease the influence of DSP,the alkali leaching of roasted kaolin was carried out with a solid/liquid ratio of 1:100 g/mL at373 K and the results are shown in Table 2.
| 图表编号 | XD0064474600 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.01 |
| 作者 | 李小斌、王洪阳、周秋生、齐天贵、刘桂华、彭志宏、王一霖 |
| 绘制单位 | 中南大学冶金与环境学院、中南大学冶金与环境学院、中南大学冶金与环境学院、中南大学冶金与环境学院、中南大学冶金与环境学院、中南大学冶金与环境学院、中南大学冶金与环境学院 |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 2 Leaching results of roasted kaolin in alkali solution”的人还看了
-
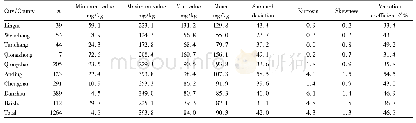
- Table 3 Distribution characteristics of soil alkali-hydrolyzable nitrogen content in the Nandu River Basin of Hainan Pro