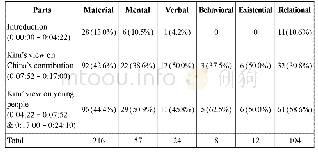
《Table 3–Mean Percentage Deviations of various solutes in water.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Model evaluation for the prediction of solubility of active pharmaceutical ingredients(APIs) to guide solid–liquid separator design》
The effect of these two assumptions is considered in this work,using benzene as a reference solvent.These results are compared in Table 2.Mishra and Yalkowsky[15]have analysed this behaviour for similar solutes,in benzene.In their work,for APIs in benzene,employing the UNIFAC combinitorial term,with the Scatchard–Hildebrand[63,64]residual term,with the assumption of zero heat capacity changes,provided the best prediction of solubility.Benzene is used as a representative solvent for all hydrophobic solvents(alkane,aliphatics,alkenes,alkynes)due to the abundance of experimental data available in the literature for pharmaceutical systems with benzene as the solvent.It is not recommended as a pharmaceutical process solvent as it is a class one residual solvent.In practice,less hazardous hydrophobic solvents such as alkanes are used.Unfortunately,the data for pharmaceutical+alkane systems for a specific alkane e.g.hexane was not abundant in the literature and so a comprehensive result regarding heat capacity assumptions would not have been possible.It is assumed that the results obtained in this work using benzene would be very similar for systems composed of other hydrophobic solvents.
| 图表编号 | XD00595300 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.05.01 |
| 作者 | Kuveneshan Moodley、Jürgen Rarey、Deresh Ramjugernath |
| 绘制单位 | Thermodynamics Research Unit, School of Engineering, University of KwaZulu-Natal, Howard College Campus、Thermodynamics Research Unit, School of Engineering, University of KwaZulu-Natal, Howard College Campus、Industrial Chemistry, Carl von Ossietzky Univer |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 3–Mean Percentage Deviations of various solutes in water.”的人还看了
-

- 表4 各类碳酸盐岩占西南喀斯特地区的面积百分比Tab.4 Percentage of various types of carbonate rocks in the karst areas of Southwest China