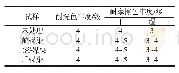
《Table 2–Kinetic equations in different conditions of dye removal.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Kinetic study on photocatalytic degradation of Acid Orange 52 in a baffled reactor using TiO_2 nanoparticles》
[AO52]:initial dye concentration;[TiO2]:amount of TiO2nanoparticles;Q:inlet flow rate;L:flow length;T:temprature;R2:R-squared;C:dye concentration during photocatalytic reaction;t:time.
According to this figure,during the complete destruction of the azo bond,benzene compounds of benzensulfonic acid,N,N-dimethylaniline and 4-dimethylamin-phenol will be obtained so that the examination of the LD50amount of these compounds in Table 1 indicates the reduced toxicity of wastewater after removing the dye agent.
| 图表编号 | XD0052059100 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.05.15 |
| 作者 | Payam Zanganeh Ranjbar、Bita Ayati、Hossein Ganjidoust |
| 绘制单位 | Civil and Environmental Engineering Faculty, Tarbiat Modares University、Civil and Environmental Engineering Faculty, Tarbiat Modares University、Civil and Environmental Engineering Faculty, Tarbiat Modares University |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 2–Kinetic equations in different conditions of dye removal.”的人还看了
-

- 表2 观测站点气温和降水的回归拟合方程Table 2 Regression fitting equations of temperature and precipitation in different observation sites
-
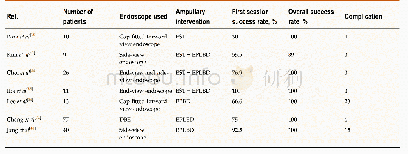
- Table 7 Success rates of stone removal in Billroth II reconstruction in different ampullary interventions
-

- Table 2 Saturated adsorption capacity of MB in dark and kinetic data of photocatalytic degradation in the presence of di