《Table 2:Electro negativity values and difference of elements[27]》
![《Table 2:Electro negativity values and difference of elements[27]》](http://bookimg.mtoou.info/tubiao/gif/ZZAF201902002_08600.gif) 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Effect of Ca on microstructure and high temperature creep properties of AM60-1Ce alloy》
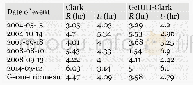
In the magnesium–aluminum–calcium ternary alloy,aluminum can affect the dissolution of calcium in the solid solution,which makes it difficult for calcium to be dissolved in theα-magnesium matrix[17].Calcium can dissolve inβ-Mg17Al12 at less than 1.0%It is easier for calcium to combine with aluminum and magnesium compared with other elements to produce intermetallics when the calcium content exceeds 1.0%.The phenomenon is related to the electronegativity difference[18-19].Usually,electronegativity is used to measure the chemical affinity.A higher electronegativity difference between two elements results in a stronger chemical affinity between them,and a more stable generated compound[20].Table 2 shows the electronegativity difference between the main elements in the alloy(Pauling’s Electronegalivity)[21].The electronegativity difference between elements is?PAl-Ca=0.6>?PMg-Ca=?PAl-Mg=0.3,which indicates that the stability of Al2Ca exceeds that of Mg2Ca or Mg17Al12.The stability of the compound can be assessed from its formation enthalpy or melting point.The?H of the three compounds Al2Ca,Mg2Ca,and Mg17Al12 were–217 kJ·mol-1[22],–39.3 kJ·mol-1[23],and–1.09 kJ·mol-1[24],and the melting points were 1,352 K,988 K,and 710 K,respectively The melting point of Al2Ca is the highest,thus,it is the easies to form and is the most stable,followed by Mg2Ca.The mos difficult to form and the least stable compound was Mg17Al12The mass ratio of Ca/Al in the magnesium–aluminum–calcium series of alloys determines the compound type[25].When the mass percentage ratio of Ca/Al is less than 0.8,an Mg2Ca compound cannot be obtained and only an Al2Ca compound formed.The mass ratio of Ca/Al in the AM60-1Ce-x Ca series alloys in this experiment were less than 0.8,so it was difficult to obtain Mg2Ca compound.In contrast,the study of AZ31magnesium alloys with calcium,strontium and cerium at the trace level(approximately 0.2wt.%per element)showed the existence of phases other than Mg17(Al,Zn)12 and Al8Mn5,namely:Al2Ca,Al2Sr,Al11Ce3,et al[26].
| 图表编号 | XD0049760500 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.03.01 |
| 作者 | Wei Jin、Yu-lai Song、Yao-hui Liu、Ping Zhao、R.D.K.Misra |
| 绘制单位 | Key Laboratory of Automobile Materials (Ministry of Education), College of Materials Science and Engineering, Jilin University、Key Laboratory of Automobile Materials (Ministry of Education), College of Materials Science and Engineering, Jilin University、K |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 2:Electro negativity values and difference of elements[27]”的人还看了
-

- Table 2 Proportion of non-adherent particles under different outgrowth values and melt delivery tube diameters