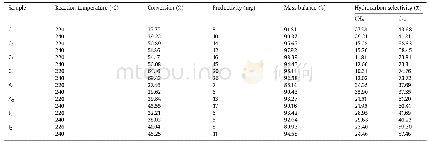
《Table 1 Rate of hydrogen production by catalysts》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《原位生成非晶纳米钴高效催化氨硼烷的醇解制氢(英文)》
aReaction condition:Reaction of anhydrous catalyst(1.2 mg)in methanol(4 m L),with addition of anhydrous AB(2 mmol)to system;TOF=TONH2/t,where t is the reaction time(h);Entire system remains 298 K.
Next,the methanolysis dehydogenation of AB catalyzed by homogeneous and heterogeneous cobalt catalysts was researched.Cobalt complexes 1~4 were chosed because they displayed high catalytic activity in the reduction reaction by AB.Co NPs and other metal NPs were easy prepared by the metal reduction by AB in-situ method.As was shown in Table 1,the Co NPs and complex 1 displayed higher performance for hydrogen production.The generated hydrogen was measured by a drainage method,using a burette to observe the volume of hydrogen.The volume of hydrogen produced versus time was plotted in Fig.2.We have proceeded the twice experiments with the methanolysis of AB catalyzed by Co NPs and complex1 respectively.Due to the similar rate and amount of hydrogen evolution,it showed good reproducibility.As the reaction gone,the structure of the complex 1might be destroyed by the AB which leaded to the decrease of hydrogen production rate.However,in-situ formed amorphous Co NPs exhibited high activity all the time and its turnover frequency(TOF)was calculated to be 515 molH2·mol-1metal·h-1during the first hydrogen release process(Fig.2).Compared with some noble metal-based catalysts[27-28,33],the catalytic performance of Co NPs was poor.However,the value of TOF was much higher than those of Cu-based catalysts,which could only reach up to 19 molH2·mol-1metal·h-1[34].Moreover,Sun et al.[35]and Filiz et al.[36]demonstrated that metallic Co was very active towards the methanolysis of AB.
| 图表编号 | XD0043292500 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.10 |
| 作者 | 陈浩、俞哲健、徐丹丹、李洋、王明明、夏良敏、罗书平 |
| 绘制单位 | 浙江工业大学绿色化学合成技术国家重点实验室培育基地、浙江工业大学绿色化学合成技术国家重点实验室培育基地、浙江工业大学绿色化学合成技术国家重点实验室培育基地、浙江工业大学绿色化学合成技术国家重点实验室培育基地、浙江工业大学绿色化学合成技术国家重点实验室培育基地、浙江工业大学绿色化学合成技术国家重点实验室培育基地、浙江工业大学绿色化学合成技术国家重点实验室培育基地 |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 1 Rate of hydrogen production by catalysts”的人还看了
-

- Table 1 Comparison of BET surface area and photocatalytic hydrogen production rate (HPR) over different CdS nanocrystals
![Table 1 Total net primary productivity of China simulated by different models[17]](http://bookimg.mtoou.info/tubiao/gif/QQSJ201901005_11900.gif)