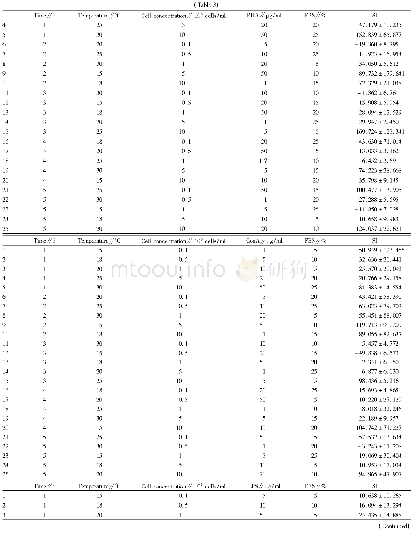
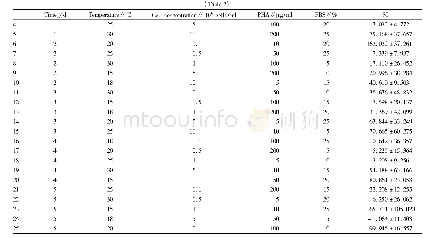
《Table 3–Results of in vitro permeation study of Lop (Preliminary study) .》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"Systematically optimized topical delivery system for Loperamide hydrochloride: Formulation design,in vitro and in vivo biopharmaceutical evaluation"》
For the 13 run formulations,the flux value and skin retention were taken as dependent variables and results were recorded in Table 4.The flux value and skin retention for the13 formulations showed a variation from 9.78±1.99μg/cm2/h to 65.48±1.67μg/cm2/h and 2.53±0.56 mg/g to 9.86±0.97 mg/g,respectively.Data was subjected to multiple regressions to yield a second-order polynomial equation.Full model of Y1and Y2were established by putting values of regression coefficients for the two response variables(Y1and Y2)respectively in Eq.1.
| 图表编号 | XD00186266500 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.05.01 |
| 作者 | Jianhui Shu、Jingjing Zhao、Fang Guo |
| 绘制单位 | Nanjing Sanhome Pharmaceutical CO.,LTD.R&D Insititute、Nanjing Sanhome Pharmaceutical CO.,LTD.R&D Insititute、Nanjing Sanhome Pharmaceutical CO.,LTD.R&D Insititute |
| 更多格式 | 高清、无水印(增值服务) |