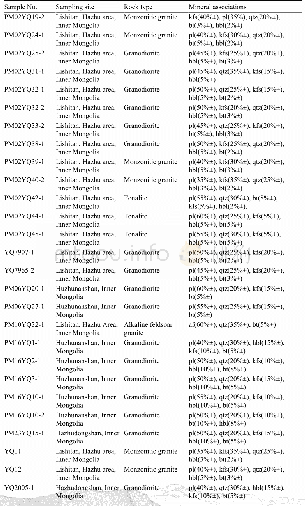
《Table 2 Amount of Lewis and Brnsted acid sites of DHBEA and HTS zeolites detected by pyridine adsor
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Environmentally-Friendly Catalytic Oxidation of Cyclohexanone with 30% H_2O_2 Solution: A Comparison Study between Hollow Titanium Silicate and Dealuminated HBEA Zeolites》
Figure 2 shows the catalytic performance of DHBEA and HTS zeolites in cyclohexanone oxidation reaction under the same conditions,with 30%H2O2 solution used as the oxidant.As shown in Figure 2(a),the maximum cyclohexanone conversion in the DHBEA zeolite catalyzed system(over 90%)is much higher than that in the HTS one(about 60%),which means that the DHBEA zeolite has higher capability for activation of cyclohexanone or H2O2 molecules during the catalytic oxidation process.Generally,the activation of substrates is dependent on the amount of acid sites,and hence the catalytic performance is in good agreement with the results of pyridine adsorbed IR analysis.Moreover,the maximum cyclohexanone conversion is obtained within0.5 h over the DHBEA zeolite,while that obtained over HTS zeolite is achieved in 2 h,respectively.It is indicated that the DHBEA zeolite has greater mass diffusion performance than HTS zeolite,which is closely related to their crystal size and microporous channels that can match well with the results shown in Figure 1 and Table 1.As illustrated in Figure 2(b),the total target product selectivity catalyzed by the DHBEA zeolite is much higher than that catalyzed by the HTS zeolite.It is inferred that many side-products are generated in the HTS zeolite catalyzed system,which is caused by the highly reactive TiOOH species(as demonstrated by many experimental and simulation researches in literature reports)[34-36].To verify this conclusion,Figure3 shows the distribution of target product selectivity as a function of reaction time in both DHBEA and HTS catalyzed systems.We can observe that a small amount ofε-caprolactone is produced at the initial stage in both systems,and it decreases gradually along with the increase in reaction time.At the same time,the selectivity of 6-hydroxyhexanoic acid,formed via the hydrolysis ofε-caprolactone,is increasing until it reaches a maximum conversion in 2 h.However,there is almost no adipic acid generated in the DHBEA catalyzed system,while the selectivity of adipic acid is over 20% in the HTS catalyzed reaction.Scheme 1 gives the reaction pathways of cyclohexanone oxidation catalyzed by the DHBEA and HTS zeolites,which reveals that the DHBEA zeolite does not cause activation on6-hydroxyhexanoic acid and H2O2 molecules without forming thereby the deep-oxidation product(adipic acid).As a result,different product distributions are observed for two zeolites,which are associated with the different mechanisms caused by various active sites.And the detailed mechanisms will be demonstrated and discussed in the next section.
| 图表编号 | XD0011308200 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.12.30 |
| 作者 | Xia Changjiu、Zhao Yi、Zhu Bin、Lin Min、Peng Xinxin、Dai Zhenyu、Shu Xingtian |
| 绘制单位 | State Key Laboratory of Catalytic Materials and Reaction Engineering, Research Institute of Petroleum Processing SINOPEC、State Key Laboratory of Catalytic Materials and Reaction Engineering, Research Institute of Petroleum Processing SINOPEC、State Key Lab |
| 更多格式 | 高清、无水印(增值服务) |