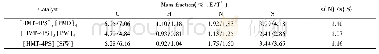
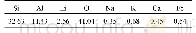《Table 3 Elemental analysis of fresh, spent and HI-regenerated catalyst samples》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Optimum Operating and Regeneration Parameters of ZnI_2 Catalyst for Converting Methanol to Triptane:An Ideal Component of Unleaded Aviation Gasoline》
In order to confirm the above postulation,elemental analyses of the fresh,spent and regenerated catalysts were carried out by XRF measurement,as shown in Table 3.It is observed that the iodine to zinc molar ratio of fresh Zn I2catalyst sample was about 1.98,which was close to the theoretic value.This ratio for the spent catalyst after six reaction cycles had dropped to 1.73,which was resulted from the iodine loss.When the spent Zn I2 catalyst was regenerated by using HI,this ratio increased once again to 2.18.It has been proved that Zn O can be soluble in HI acid.In other words,HI was able to react with Zn O and could convert it into Zn I2.Therefore,it is suggested that a certain amount of Zn O was formed and a part of iodine species was lost during the catalytic reaction and there would be two causes leading to deactivation.
| 图表编号 | XD0011302200 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.06.30 |
| 作者 | Chen Weiwei、Song Yueqin、Zong Rui、Du Changfei、Zhou Xiaolong、Li Chenglie |
| 绘制单位 | Department of Petroleum Processing,East China University of Science and Technology、Department of Petroleum Processing,East China University of Science and Technology、Department of Petroleum Processing,East China University of Science and Technology、Beijin |
| 更多格式 | 高清、无水印(增值服务) |