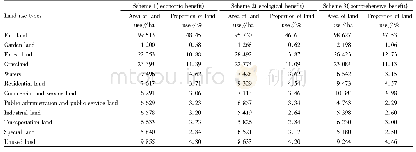
《Table 1Grouping scheme used in acute toxicity assay.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Synergistic effect and different toxicities of adjuvant components of Realgar–Indigo Naturalis formula》
a BC=acute toxicity assay of blank control group,‘a’=‘acute’.Same as below.
The animals were divided into 21 groups of ten animals each(five males and five females).The scheme used for grouping was shown in Table 1.The doses of Realgar used in each group were designed according to the results of a pilot study.All treatments were administered once by oral gavage.The animals were closely observed for 4 h following administration and then once daily for14 d to evaluate general behavior,clinical signs of toxicity and mortality.Body weights were measured before and after administration and on days 7 and 14.At the end of the experiment,the animals were anesthetized using pentobarbital sodium(45 mg/kg i.p.).After the animal was deeply anesthetized,it was euthanized via cervical dislocation,and the following organs were removed for histopathological analysis:the brain,heart,lungs,liver,stomach,small intestine(section),testicle(ovarian),and kidneys.
| 图表编号 | XD007991600 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.04.18 |
| 作者 | Huan-Hua Xu、Zeng-Chun Ma、Qiao-Li Shi、Shi-Han Yang、La Jiang、Xiang-Mei Chen、Yue Gao |
| 绘制单位 | Guangdong Pharmaceutical University、Institute of Radiation Medicine, Academy of Military Medical Sciences、Guangdong Pharmaceutical University、Institute of Radiation Medicine, Academy of Military Medical Sciences、State Key Laboratory of Drug Metabolism, He |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 1Grouping scheme used in acute toxicity assay.”的人还看了
-

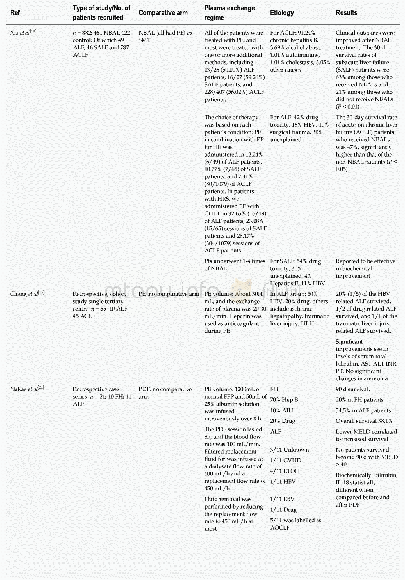
- Table 5 Studies included for use of plasmapheresis in acute liver failure and acute-on-chronic liver failure in adults
-
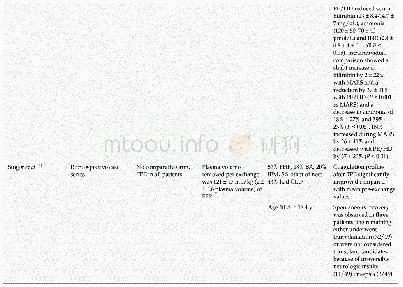
- Table 5 Studies included for use of plasmapheresis in acute liver failure and acute-on-chronic liver failure in adults