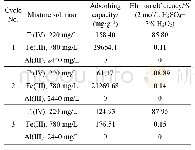
《Table 5 Performance of CA-P204 packed into fixed-bed column for three consecutive adsorption–elutio
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《浸渍二(2-乙基己基)磷酸的海藻酸钙微球去除和回收高硫铝土矿浸出液中的微量钛(Ⅳ)(英文)》
In order to improve the removal efficiency of Ti(IV)from high concentration Al(III)(CAl=2440 mg/L) and Fe(III)(CFe=780 mg/L) leaching solution and examine the reusability of the prepared adsorbent,three successive adsorption–desorption cycles were performed using a glass column with an amount of the CA-P204 as adsorbent(the experimental installation is shown in Fig.6 (a)) .As shown in Table 5,Al(III)was not adsorbed on the CA-P204,and a certain amount of Ti(IV)and Fe(III)were adsorbed simultaneously.Although some iron was co-adsorbed by CA-P204,6 mol/L HCl was used to strip Fe(III)before stripping Ti(IV)(the adsorption-desorption cycle process chart is shown in Fig.6(b)) .The maximum adsorption capacities of Ti(IV)on the CA-P204 adsorbent for three successive column cycles were calculated as 158.40,61.17,and124.93 mg/g,respectively.The second adsorption amount of adsorbents for Ti(IV)was much smaller than that of the first and third adsorption.The possible reason is that when the leaching solution flows through the column,Ti(IV)is adsorbed initially at the top of column As the volume of the leaching solution increases,the Ti(IV)adsorbed on the head of column further increases and a portion of Ti(IV)is likely to enter into the internal structure of the adsorbent,which is not easy to elute This leads to the reduction of the adsorption site,so the adsorption capacity of the second adsorption is less than those of the other two cycles.
| 图表编号 | XD0064474300 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.01 |
| 作者 | 娄振宁、肖欣、熊英、翟玉春 |
| 绘制单位 | 东北大学冶金学院、辽宁大学化学院、辽宁大学化学院、辽宁大学化学院、东北大学冶金学院 |
| 更多格式 | 高清、无水印(增值服务) |