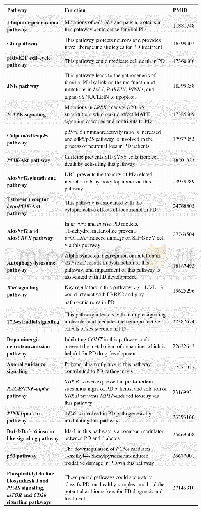
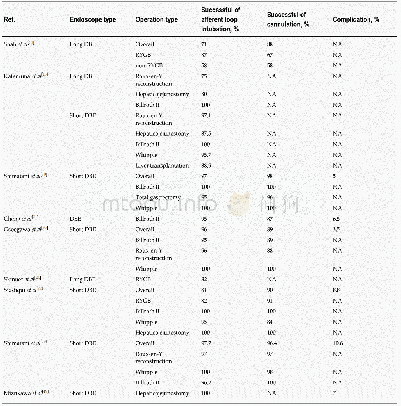
《Table 2 Biological, pharmacokinetic, clinical and endoscopic data at baseline and during follow up》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Endoscopic response to tumor necrosis factor inhibitors predicts long term benefits in Crohn's disease》
IQR:Interquartile range;CRP=C reactive protein;TNF-alpha:Tumor necrosis factor alpha;ATIs:Anti-infliximab antibodies;ATAs:Anti-adalimumab antibodies;CDAI:Crohn’s Disease Activity Index;CDEIS:Crohn’s Disease Endoscopic Index of Severity.
Demographic and baseline disease characteristics are summarized in Table 1.A majority of patients received combination therapy(86%).The proportion of patients achieving MH at week 46 under IFX and ADA were similar(46%vs 42%),the subsequent analysis was therefore performed in the pooled population.
| 图表编号 | XD0064281800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.04.14 |
| 作者 | Ignacio Alfaro、Maria Carme Masamunt、Nuria Planell、Alicia López-García、Jesús Castro、Marta Gallego、Rebeca Barastegui、Angel Giner、Alejandro Vara、Azucena Salas、Elena Ricart、Julián Panés、Ingrid Ordás |
| 绘制单位 | Gastroenterology Department, Hospital Clínic de Barcelona、Gastroenterology Department, Hospital Clínic de Barcelona、Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), University of Barcelona、Gastroenterology Department, Hospital Clínic d |
| 更多格式 | 高清、无水印(增值服务) |