《Table 3–Comparison of aluminum sulphate and polyaluminium chloride.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Investigating the coagulation of non-proteinaceous algal organic matter: Optimizing coagulation performance and identification of removal mechanisms》
Difference in the lowest Al residual concentrations achieved for alum and PACl(see Table 3)also stem from different Al species distribution for alum and PACl.According to Wang et al.(2014)and Van Benschoten and Edzwald(1990),at pH values of the lowest Al residuals in this study5%‐10%of dosed Al may be present in the form of Ala.High residual Al concentrations may thus be expected,and the portion of Alafor PACl is higher than for alum.This is in accordance with our results and explains higher residual Al concentrations for PACl than for alum achieved in our experiments.Moreover,different Al species distribution for alum and PACl is probably the reason for different efficiencies in removing various MW fractions by alum and PACl,as shown in Section 2.2(Fig.1b,c).Highly stable Alb(mostly Al13)species of PACl are able to interact with lower-MW fractions through charge neutralization(Yan et al.,2007)Therefore,PACl was more efficient in removing 3–10,10–30and 30–50 kDa fractions than alum.
| 图表编号 | XD0052051800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.05.15 |
| 作者 | Jana Naceradska、Katerina Novotna、Lenka Cermakova、Tomas Cajthaml、Martin Pivokonsky |
| 绘制单位 | Institute of Hydrodynamics of the Czech Academy of Sciences、Institute of Hydrodynamics of the Czech Academy of Sciences、Institute of Hydrodynamics of the Czech Academy of Sciences、Institute of Microbiology of the Czech Academy of Sciences、Institute of Hyd |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 3–Comparison of aluminum sulphate and polyaluminium chloride.”的人还看了
-
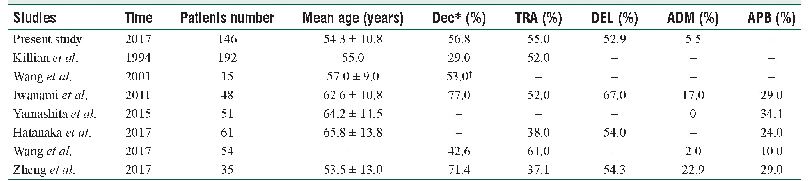
- Table 3:Comparison of previous reports and the present study on frequency and distribution of decremental responses in p