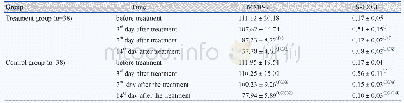
《Table 1 Detailed property ofβ-C2S before and after carbonation》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Microstructure of β-Dicalcium Silicate after Accelerated Carbonation》
Fig.6 depicts nitrogen adsorption-desorption isotherms and the corresponding pore size distributions ofβ-C2S before and after carbonation.The detailed properties of the samples are listed in Table 1.From? Fig.6(a)and Table 1,it is revealed that theβ-C2Sbefore carbonation has a very small surface area(2.455m2/g),substantially non-porous structure.However,the surface area(4.214 m2/g)and pore volume ofβ-C2S are all enlarged noticeably after carbonation.The isotherms ofβ-C2S after carbonation exhibit a type of IV according to the IUPAC.The well-defined hysteresis loops with a steep desorption branch and less steep adsorption branch belong to the H2a-type,which is typical for a three-dimensional hierarchical scaffold-like mesoporous structure.The formation of this mesoporous structure inβ-C2S after carbonation is attributed to the three-dimensional network silica gels formed during the carbonation.The pore size distribution curves are shown in Fig.6(b).Before carbonation,the pore size distribution was centered at 0.614 and 15.34 nm,which are attributed to the interstitial and space inβ-C2S.Interestingly,the pore size distribution curve ofβ-C2S after carbonation centered at 1.75,4.75,7.71,10.77,28.92 nm and36.08 nm was observed.Based on the results discussed above,the enlarged pore from 0.614 to 1.75 nm is caused by the decalcification process ofβ-C2S induced by carbonation.The pore size distribution centered at 4.75,7.71,and 10.77 nm is attributed to the threedimensional network silica gels and the pores centered at 28.92 and 36.08 nm are postulated to be the interface gap between hierarchically distributed carbonation products and un-carbonatedβ-C2S.
| 图表编号 | XD0043327400 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.01 |
| 作者 | 刘松辉、WANG Junjie、张海波、GUAN Xuemao、QIU Man、DOU Zhenzhen |
| 绘制单位 | School of Materials Science and Engineering, Henan Polytechnic University、State Key Laboratory of Green Building Materials, China Building Materials Academy、School of Materials Science and Engineering, Henan Polytechnic University、School of Materials Scie |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 1 Detailed property ofβ-C2S before and after carbonation”的人还看了
-

- Table 2 Changes in main chemical properties of alkaline-saline soil before and after soil improvement