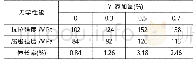
《Table 2Textural properties of HCh Cl‐Y, HCh OH‐Y and HNa Y samples before and after the hydrotherma
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《以胆碱为绿色无毒有机结构导向剂合成高硅Y型分子筛(英文)》
a Estimated by the reflection intensities of the X‐ray diffraction peaks(1 1 1),(2 2 0) and(3 3 1)of the samples.
It is well known that the(hydro)thermal stability of zeolite at high temperature has a strong relationship with the frame‐work Si O2/Al2O3 ratio,which is a crucial factor for industrial applications.The exothermic peaks without weight variation above 800°C in the TG/DSC curves are attributed to the struc‐tural collapse of zeolite Y,which is used as a main evaluation of the thermal stability of zeolites at high temperature.Na‐Y starts to collapse at 970°C(Fig.S7),but the skeleton of ChCl‐Y and Ch OH‐Y remains stable until 1024 and 1030°C(Fig.2E and 2F).The results are also additional evidence that our materials have unusually ultrahigh Si O2/Al2O3 ratio.The crystallinity change before and after steam treatment at high temperature is a measure of the hydrothermal stability of zeolites.The XRD pat‐terns of HChCl‐Y,HChOH‐Y and HNa‐Y(the correspond H‐form samples after ammonium ion exchange and calcination)before and after 100%steam treatment at 750°C for 2 h are shown inFig.5.This indicates that the crystallinity of HNa‐Y descents more steeply than that of HCh Cl‐Y and HCh OH‐Y after steam treatment.The crystallinity of HCh Cl‐Y and HCh OH‐Y retains54.2%and 56.6%,higher than 38.5%of HNa‐Y;BET specific surface area and t‐plot micropore surface area of HNa‐Y are obviously lower than that of HChCl‐Y and HChOH‐Y after steam treatment(Table 2).The results indicate that Ch Cl‐Y and Ch OH‐Y exhibit a more superior hydrothermal and thermal stability owing to the higher framework SiO2/Al2O3 ratio.It is known that the bond energy of the silicon–oxygen bond(Si–O–Si)is higher than that of the aluminium–oxygen bond(Al–O–Si),so the Si–O–Si bond is more stable than the Al–O–Si bond.Ch Cl‐Y and Ch OH‐Y have higher Si–O–Si/Al–O–Si ratio than Na‐Y,which has been interpreted with the 29Si‐NMR spec‐trum(Fig.4).Collectively,these studies confirm the practical advantages of increasing Y zeolite SiO2/Al2O3 ratio to improve the(hydro)thermal stability,which enhances the adaptation of Y zeolite for catalytic applications.
| 图表编号 | XD0028863800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.01 |
| 作者 | 贺大威、袁丹华、宋智甲、徐云鹏、刘中民 |
| 绘制单位 | 中国科学院大连化学物理研究所洁净能源国家实验室(筹)甲醇制烯烃国家工程实验室、中国科学院大学、中国科学院大连化学物理研究所洁净能源国家实验室(筹)甲醇制烯烃国家工程实验室、中国科学院大连化学物理研究所洁净能源国家实验室(筹)甲醇制烯烃国家工程实验室、中国科学院大学、中国科学院大连化学物理研究所洁净能源国家实验室(筹)甲醇制烯烃国家工程实验室、中国科学院大连化学物理研究所洁净能源国家实验室(筹)甲醇制烯烃国家工程实验室 |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 2Textural properties of HCh Cl‐Y, HCh OH‐Y and HNa Y samples before and after the hydrothermal treatment by 100%st”的人还看了
-
- 表3 不同Y添加量下铸造Al-30Si合金的力学性能Tab.3 Mechanical properties of Al-30Si casting alloy with different Y contents
-

- 表4 不同热处理条件下的AZ91D-1.5%Y镁合金力学性能Tab.4 Mechanical properties of AZ91D-1.5Y magnesium alloy under different heat treatment c





