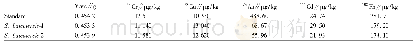《Table 1 Some key scientific questions of primate pluripotent stem cells.The labels“+”indicate that
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Primate stem cells: bridge the translation from basic research to clinic application》
The gold standard for assessing pluripotency is the ability to generate chimeras,in which donor cells introduced into preimplantation embryos integrate into the entire fetus,thereby enabling a broad assessment of donor cell developmental capacity(Mascetti and Pedersen,2016).Our previous studies have shown that cynomolgus monkey ESCs grown in NHSMV culture conditions(Gafni et al.,2013)are able to incorporate into host embryos and develop into chimeras with contribution in all three germ layers as well as in germ cell progenitors(Chen et al.,2015).Our data shows that it is feasible to generate chimeric monkeys using ESCs and this finding opens up new avenues for the use of NHP models to study both pluripotency and human disease.However,the transcriptional analysis indicated that ESCs grown in NHSMV culture conditions are closer to post-implantation EPI cells(Nakamura et al.,2016),suggestive of species differences.Despite these and other advances,two burning questions still remain(Table 1):(i)whether authentic naive primate ESCs with the ability to produce viable progenies can be captured and cultured in vitro by improving the culture system;(ii)what are key mechanisms regulating primate PSC plasticity and maintaining naive pluripotency?The proposition of a naive state residing within the primate embryo does not imply that primate naive pluripotency simply replicates the rodent paradigm.The depth analysis of the primate embryo development would reveal the distinguishing features of pre-EPI cells and eventually help us to define and isolate naive PSCs.In addition,pluripotent cell isolation and state transitions between naive and primed states provide an in vitro model system to examine the very early steps of human epiblast development and enrich our knowledge of the signaling,genetic,epigenetic,and metabolic pathways during this process(Wu and Izpisua Belmonte,2016).
| 图表编号 | XD0034726000 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.05 |
| 作者 | Tianqing Li、Zongyong Ai、Weizhi Ji |
| 绘制单位 | Yunnan Key Laboratory of Primate Biomedical Research, Institute of Primate Translational Medicine, Kunming University of Science and Technology、Yunnan Key Laboratory of Primate Biomedical Research, Institute of Primate Translational Medicine, Kunming Univ |
| 更多格式 | 高清、无水印(增值服务) |