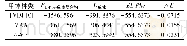《Table 2 Interaction energies of ILs-DCM》
To further uncover the absorption mechanism of DCM by ILs,the interactions between cations or anions and DCM were investigated by quantum chemistry calculations at the level of B3LYP/6-311++G**with Gaussian 09 software.The interaction structures of cation,anion and IL-DCM were adjusted to the optimum structure and interaction energies of cations,anions and IL-DCM were displayed in Tables 2 and 3.The total interaction energies of[Bmim][SCN],[Bmim][DCA],[Bmim][BF4],[Bmim][PF6],[Bmim][NTf2]with DCM were–48.82,–45.31,–42.18,–41.26,–32.14 kJ/mol,respectively,followingtheorderof[Bmim][SCN]>[Bmim][DCA]>[Bmim][BF4]>[Bmim][PF6]>[Bmim][NTf2],which was in good agreement with the order of the absorption performance of imidazolium-based ILs.According to the results of optimum structure between[Bmim][SCN]and DCM,the distances of H and N(0.203 nm)is shorter than Van der Waals distance of 0.267 nm between[SCN]–and DCM[32].Furthermore,it showed that anion had a stronger interaction with DCM considering monomer interaction energies,because of hydrogen bonds between anion and DCM.And it further confirms that the anions had a primary effect on the absorption performance and the optimum interaction structure showed in Fig.8.
| 图表编号 | XD0033430200 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.01 |
| 作者 | 吴文亮、李涛、高红帅、尚大伟、涂文辉、王斌琦、张香平 |
| 绘制单位 | 郑州大学化工与能源学院、中国科学院过程工程研究所离子液体清洁过程北京市重点实验室多相复杂系统国家重点实验室、郑州大学化工与能源学院、中国科学院过程工程研究所离子液体清洁过程北京市重点实验室多相复杂系统国家重点实验室、中国科学院过程工程研究所离子液体清洁过程北京市重点实验室多相复杂系统国家重点实验室、中国科学院过程工程研究所离子液体清洁过程北京市重点实验室多相复杂系统国家重点实验室、中国科学院过程工程研究所离子液体清洁过程北京市重点实验室多相复杂系统国家重点实验室、郑州大学化工与能源学院、中国科学院过程工程 |
| 更多格式 | 高清、无水印(增值服务) |
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式