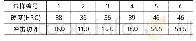《Tab.2Electrochemical properties and triplet energy of SFXPz》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"吩噻嗪功能化的螺-9,9'-氧杂蒽芴的设计、合成及其光电性质与分子结构间的相互关系研究(英文)"》
The influence of carrier transporting groups on the frontier molecular orbitals(FMOs)was further researched by density functional theory(DFT)calculations,and the relevant data were listed in Tab.1.The HOMO and LUMO energy levels are-4.89 e V and-1.21 e V,respectively,which are mainly localized on phenothiazine and fluorene,respectively.The complete separation of HOMO and LUMO between phenothiazine and fluorene is helpful to efficient hole-and electron-transporting characteries,and prevent the reverse energy transfer[23].In addition,the electrochemical properties of SFXPz were further studied by cyclic voltammetry measurements(Tab.2 and Fig.4).The HOMO(-6.15 eV)and LUMO(-1.82 eV)were estimated from the oxidation and reduction threshold of the compound.The energy gap(Eg,4.33 eV)was calculated from the difference of HOMO and LUMO.Compared to the corresponding data of SFX-Cz based our previous work[17],HOMO and LUMO of the compound are decreased and rised,respectively.Consequently,an enlarged Egis realized,which is necessary to construct high-efficient blue PhOLEDs[19].
| 图表编号 | XD0030818700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.01 |
| 作者 | 赵祥华、王莉敏、马晓、许文娟、陈明、袁顺东、徐志杰 |
| 绘制单位 | 信阳师范学院化学化工学院、有机电子与信息显示国家重点实验室培育基地信息材料与纳米技术研究院江苏先进生物与化学制造协同创新中心南京邮电大学、信阳师范学院化学化工学院、信阳师范学院化学化工学院、有机电子与信息显示国家重点实验室培育基地信息材料与纳米技术研究院江苏先进生物与化学制造协同创新中心南京邮电大学、信阳师范学院化学化工学院、中国石油大学(华东)理学院、中国石油大学(华东)理学院 |
| 更多格式 | 高清、无水印(增值服务) |