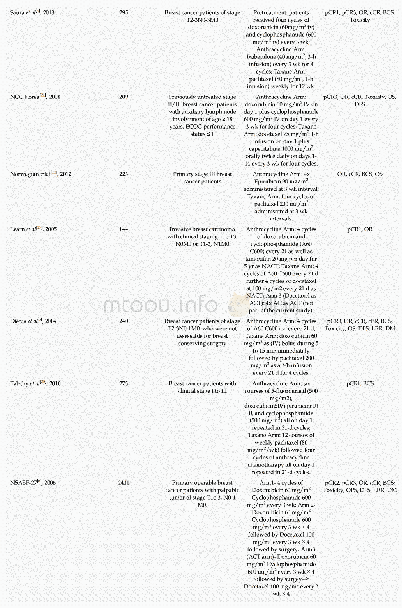
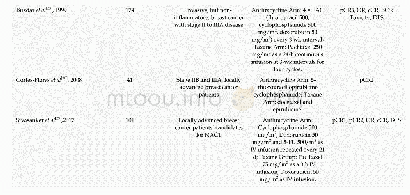
《Table 2Clinical outcomes in the intention-to-treat population.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《特力阿扎维林治疗新冠病毒肺炎的疗效与安全性——一项随机对照试验》
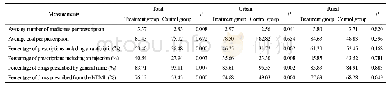
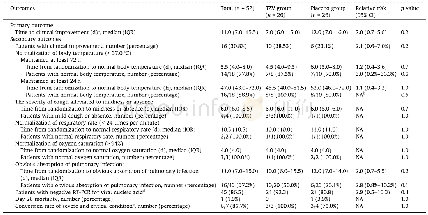
The median time to absorption of pulmonary infection was 10 d versus 12 d (RR,2.0;95%CI,0.7–5.5;p=0.2;Table 2,Fig.2(b)).Tenpatients of the TZV group and six patients in the placebo group demonstrated significant absorption of pulmonary infection(50.0%versus 26.1%,RR,2.8;95%CI,0.8–10.2;p=0.1).The negativity conversion rate from a positive COVID-19 nucleic acid test to a negative result was 92.3%versus 80.8%,with p=0.4(Table 2,Fig.2(c)).One patient who had been randomly assigned to the placebo group died over the course of the study (Table 2).There was no significant difference in the conversion rate fromsevere and critical condition to mild or ordinary condition betweenthe two groups (100.0%versus 75.0%,p=1.0).
| 图表编号 | XD00195693500 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2020.10.01 |
| 作者 | 吴效科、于凯江、王永晨、徐万海、马红丽、侯艳、李悦、蔡本志、朱丽影、张敏、胡晓丽、高敬书、王宇、秦慧超、王文杰、赵鸣雁、吴霞、张勇、李璐、李康、杜智敏、Ben Willem J.Mol、杨宝峰 |
| 绘制单位 | College of Pharmacology, Harbin Medical University、Heilongjiang Provincial Hospital, Harbin Institute of Technology、First Affiliated Hospital, Heilongjiang University of Chinese Medicine、First Affiliated Hospital, Harbin Medical University、Second Affiliat |
| 更多格式 | 高清、无水印(增值服务) |