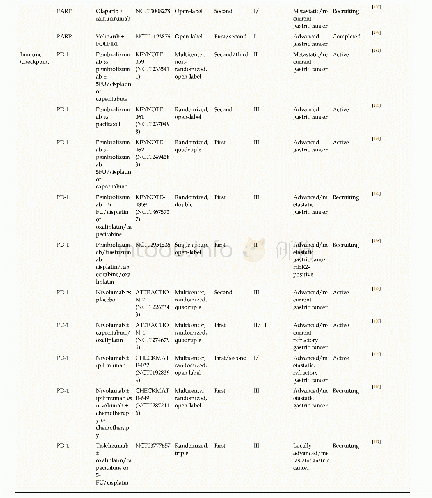
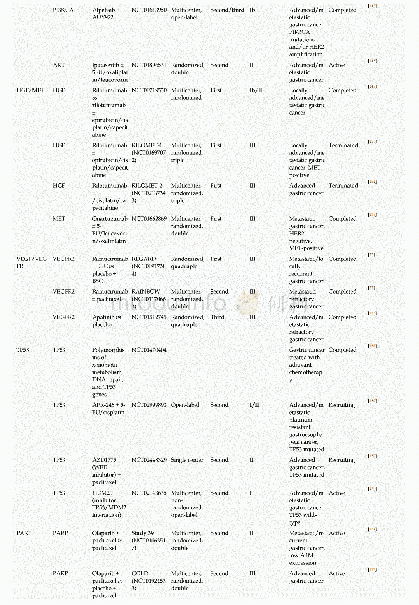
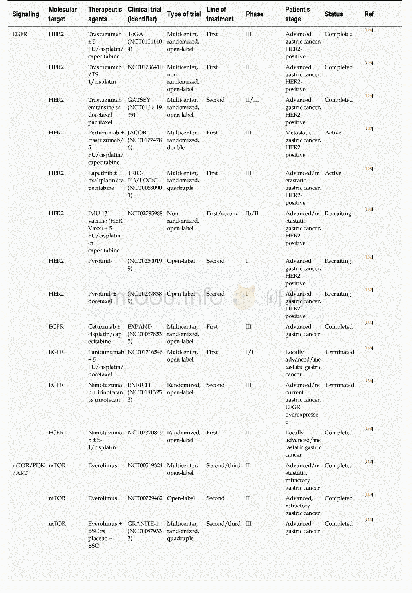
《Table 1Outcomes adopted in the protocols of clinical trials on COVID-19.》
The consensus meeting was held on 24 February 2020,and involved 20 participants.These included representatives from various stakeholder groups who were experts in respiratory,critical,TCM,and evidence-based medicine;clinical pharmacology;and statistics,in addition to medical journal editors and decisionmakers.There was no conflict of interest among the different stakeholders.Before discussion on each outcome,the results of the two rounds of Delphi survey were shown to the participants of the meeting.Based on the Delphi survey results and discussion,the participants voted anonymously for each outcome,following the criteria of clinical value,clinical feasibility,and stability ofindicators in different classifications of COVID-19.Each outcome that met the consensus standards was included in the final COS.At the end,the COS-COVID consisted of one outcome for mild(time to 2019 novel coronavirus (2019-n Co V)reverse transcription-polymerase chain reaction(RT-PCR)negativity),four outcomes for ordinary (length of hospital stay,composite events,score of clinical symptoms,and time to 2019-n Co V RT-PCR negativity),five outcomes for severe (composite events,length of hospital stay,arterial oxygen partial pressure (Pa O2)/fraction of inspired oxygen(Fi O2),duration of mechanical ventilation,and time to 2019-n Co V RT-PCR negativity),one outcome for critical (all-cause mortality),and one outcome for rehabilitation period (pulmonary function),as shown in Table 5.
| 图表编号 | XD00195687400 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2020.10.01 |
| 作者 | 金鑫瑶、庞博、张俊华、刘清泉、杨忠奇、封继宏、刘学政、张磊、王保和、黄宇虹、Alice Josephine Fauci、马玉玲、Myeong Soo Lee、元唯安、谢雁鸣、唐健元、高蕊、杜亮、张硕、祁寒梅、孙宇、郑文科、杨丰文、蔡慧姿、王可仪、欧益、黄明、朱彦、喻佳洁、田金徽、赵敏、胡镜清、姚晨、李幼平、张伯礼 |
| 绘制单位 | Evidence-Based Medicine Center, Tianjin University of Traditional Chinese Medicine、Chinese Clinical Trials Core Outcome Set Research Center、Evidence-Based Medicine Center, Tianjin University of Traditional Chinese Medicine、Chinese Clinical Trials Core Out |
| 更多格式 | 高清、无水印(增值服务) |
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式