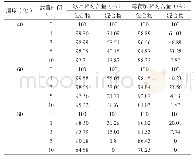
《Table 1–Apparent stability constant (Ks) of CA inclusion complexes.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Effect of antioxidant activity of caffeic acid with cyclodextrins using ground mixture method》
Phase solubility studies were conducted to assess the molar ratio of the CA/CD inclusion complexes and their stability constants in an aqueous solution.Results of the solubility testing indicated that the solubility of CA increased linearly forαCD andβCD,producing an ALtype of phase solubility diagram according to the classification system of Higuchi and Connors[19],(Fig.2) .Typically,an ALdiagram indicates that a complex is formed at a molar ratio of 1/1[20],so both CA/αCD and CA/βCD formed a complex at a molar ratio of 1/1 in solution.The stability constant(Ks)was determined next.Ks was 1547.5 M-1forαCD and 371.4 M-1forβCD(Table 1).A study by Iacovino et al.reported that,when a complex has a stability constant exceeding 1000 M-1,the guest molecule and CD are unlikely to dissociate[21].CA included inαCD had a stability constant of1547.5 M-1,so CA was more stable inside the cavity ofαCD than it was inside the cavity ofβCD.
| 图表编号 | XD00186252200 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.01.01 |
| 作者 | Ryota Shiozawa、Yutaka Inoue、Isamu Murata、Ikuo Kanamoto |
| 绘制单位 | Faculty of Pharmacy and Pharmaceutical Sciences, Josai University、Faculty of Pharmacy and Pharmaceutical Sciences, Josai University、Faculty of Pharmacy and Pharmaceutical Sciences, Josai University、Faculty of Pharmacy and Pharmaceutical Sciences, Josai Un |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 1–Apparent stability constant (Ks) of CA inclusion complexes.”的人还看了
-

- 表7 高湿对包合物和混合物稳定性的影响Tab.7 Effect of high humidity on the stability of inclusion compound and physical mixtures
-

- 表5 室温对包合物和混合物稳定性的影响Tab.5 Effect of room temperature on the stability of inclusion compound and physical mixtures