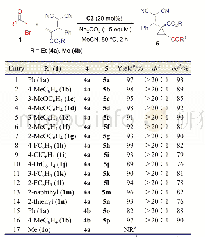
《Table 4 Catalytic asymmetric synthesis of vinylcyclopropanes5 a》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"基于α-溴代酮的催化不对称合成3,3-螺环丙基吲哚酮和乙烯基环丙烷(英文)"》
a For reaction conditions,see Experimental Section.b Isolated yield.c Only single diastereomer was observed by NMR assay.d Determined by chiral HPLC.e No reaction.
With this positive result in hand,we then attempted to survey the catalytic asymmetric version of this reaction under pre-determined conditions by choosing 1a and 2a as the model substrates(Table 1).First,the racemic model reaction catalyzed by DABCO(20 mol%)was tried,but failed to deliver the product rac-3a in appreciable yield(Table 1,Entry 1).To our delight,further chiral catalyst screening brought encouraging results.Under similar conditions,a series of cinchona alkaloids and their derivatives proved to be effective catalysts,producing 3a in modest to excellent enantioselectivities(Table 1,Entries 2~10).Quinidine(C2)and its derivatives(C1,C3~C6)all furnished moderate to high ee values although some of them only brought low yields(Table 1,Entries 2~7).Methylated quinidine(C3)showed good catalytic activity and excellent enantioselectivity,delivering 3a in 77%yield with 93%ee(Table 1,Entry 4).Cinchonine(C7)also showed good catalytic activity but only modest enantiose-lectivity(Table 1,Entry 8).Both quinine(C8)and cinchonidine(C9)were effective,giving the opposite enantiomer of 3a as the major but in moderate yields and modest enantioselectivites(Table 1,Entries 9,10).By choosing C3 as the catalyst,several solvents were further surveyed under lowered temperatures and elongated times(Table 1,Entries 11~16).With regard to both yield and enantioselectivity,toluene was selected as the preferred solvent(Entry 17).The reduced loading amount(10 mol%)of the catalyst was also surveyed,but resulted in inferior yield andlowered enantioselectivity(Entry 18).Thus,the optimal conditions for the model reaction was established as shown in Table 1 Entry 17,under which chiral spirocyclopropyl oxindole 3a was obtained in 87%yield with 95%ee.
| 图表编号 | XD00140732000 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2020.01.01 |
| 作者 | 罗京华、耿玮笙、曹仕轩、贺峥杰 |
| 绘制单位 | 南开大学化学学院元素有机化学国家重点实验室、南开大学化学学院元素有机化学国家重点实验室、南开大学化学学院元素有机化学国家重点实验室、南开大学化学学院元素有机化学国家重点实验室、天津化学化工协同创新中心 |
| 更多格式 | 高清、无水印(增值服务) |

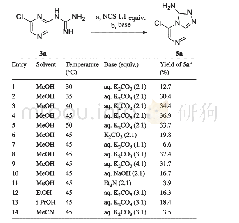
![Table 3 Cyclization reaction in the synthesis of[1, 2, 4]triazolo[4, 3-a]pyrazin-3-amines 5](http://bookimg.mtoou.info/tubiao/gif/TJDY201902009_09800.gif)
