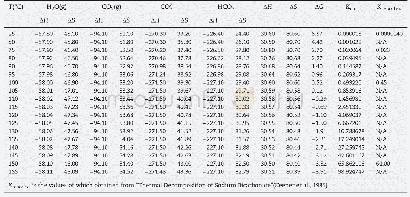
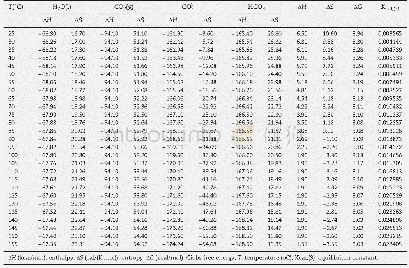
《Table 1Calculated equilibrium lattice constant a0, volume V0, formation enthalpy, H bulk modulus B0
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《第一性原理研究Al-Y合金在压力作用下的晶胞结构、力学性质、热力学性质和电子结构(英文)》
The calculated Al-Y alloy phases in this paper are cubic crystals,for which the elastic constants are C11,C12 and C44.The following are the associated conditions of mechanical stability[26]:C44>0,C11>|C12|,C11+2C12>0.Table 2 lists the calculated results for the elastic constants for three phases at zero pressure,along with experimental[20,25]and theoretical values[6,7,9].The data demonstrate that Al Y,Al2Y and Al3Y easily fit the conditions for mechanical stability,and that there is good correspondence between the results of elastic constant calculations and the available theoretical and experimental data.Thus,the calculated elastic constants and conditions as selected should be appropriate.Fig.3a,which shows changes in the elastic constants under pressure,indicates that C11,C12 and C44 increase as the pressure increases,and that C11 is more likely to change under pressure than C12 and C44 because C 11 experiences length elasticity,while C12 and C44 experience shape elasticity,which makes them susceptible to a change in shape due to transverse strain,but not to a change in volume[8].Therefore,C12 and C44 are less impressible compared with C11.
| 图表编号 | XD0011237500 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.05.01 |
| 作者 | 牛晓峰、黄志伟、王涵、胡磊、王宝健 |
| 绘制单位 | 太原理工大学材料科学与工程学院、太原理工大学先进镁基材料山西省重点实验室、中国兵器工业第五九研究所、太原理工大学材料科学与工程学院、太原理工大学先进镁基材料山西省重点实验室、太原理工大学材料科学与工程学院、太原理工大学先进镁基材料山西省重点实验室、太原理工大学材料科学与工程学院、太原理工大学先进镁基材料山西省重点实验室 |
| 更多格式 | 高清、无水印(增值服务) |