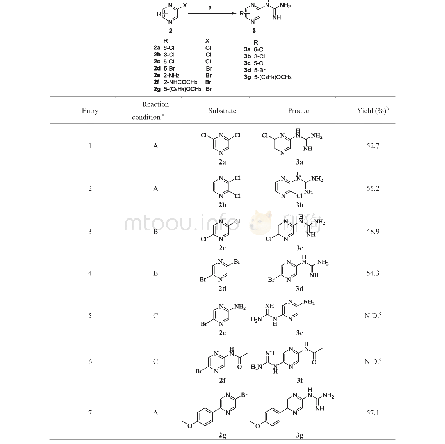
《Table 2 Preparation of 1- (pyrazin-2-yl) guanidine derivatives 3》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"Facile Synthesis of [1,2,4]Triazolo[4,3-a]pyrazin-3-amines via Oxidative Cyclization of 1-(Pyrazin-2-yl)guanidine Derivatives"》
a Reaction conditions described in the table were as follows:A.Guanidine carbonate(3 equiv.),K 2 CO 3(5 equiv.),NMP,130°C;B.Guanidine carbonate(3 equiv.),NaHCO 3(5 equiv.),NMP,120°C;C.A strong base NaOH(5 equiv.)was used,and the temperat
With these promising results in hand,we performed the optimization procedure of the cyclization reaction with various parameters,including temperature,solvent,and base,the results of which are provided in Table 1.From the table,we can see that the yield of 5a increases as the temperature increases from 30–45°C while maintaining the same alkali ratio and solvent(entries 1 to 4).A further increase in temperature results in a decrease in yield(entry 5).By changing the alkali ratio while keeping the same solvent and temperature,we observed an increasing yield trend up to a 3.1 equivalent of aq.K 2 CO 3(entries 7,4,and 8)and then a decrease in yield with a further increase in the alkali ratio(entry 9).By replacing the solvent and base entity with those used in our aforementioned study,an unsatisfactory yield often results for 5a,with less than 20%(entries 6 and10–14).We achieved a maximum yield of 37.4%by maintaining a 3.1 equiv.of aq.K 2 CO 3(5 mL H 2 O)in methanol at 45°C.
| 图表编号 | XD0076873200 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.04.01 |
| 作者 | Wei Li、Jieqiong Kang、Xueqin Zhou、Dongzhi Liu、Haiya Sun、Fang Xu、Tianyang Wang |
| 绘制单位 | School of Chemical Engineering and Technology,Tianjin University、Collaborative Innovation Center of Chemical Science and Engineering、Tianjin Engineering Research Center of Functional Fine Chemicals、School of Chemical Engineering and Technology,Tianjin Uni |
| 更多格式 | 高清、无水印(增值服务) |





