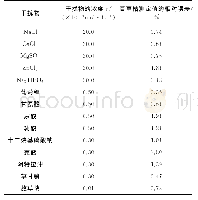
《Table 1–Results of irinotecan hydrochloride (nano) micelle interference test.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《LAL test and RPT for endotoxin detection of CPT-11/DSPE-mPEG_(2000) nanoformulation: What if traditional methods are not applicable》
The results are listed in Table 1.For both LAL reagents(manufactured by Zhanjiang A&C and Xiamen Limulus Reagent Company),the endotoxin free water showed negative results and the endotoxin positive controls(Group 1)showed the sensitivity of detection ES=λ,indicating that LAL results are valid.However,for samples 20150325DM and 20150427DM,the two kinds of LAL reagents behaved significantly differently.The results from LAL reagent manufactured by Zhanjiang A&C Biological Co.,Ltd.showed that with 20150427DM,the sensitivity of detection Etis not between 0.25λ-2λ,which indicated that0.125 mg/ml sample concentration causes interference for the reaction between LAL reagents and endotoxin.On the other hand,the LAL reagent manufactured by Xiamen Limulus Reagent Experimental Factory Co.,Ltd.generated all positive results,including negative controls of samples 20150325DM and20150427DM.These results demonstrated that at a drug concentration of 0.125 mg/ml,the irinotecan hydrochloride(nano)micelle interferes with the LAL test.
| 图表编号 | XD00596300 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.05.01 |
| 作者 | Yanan Jin、Juanjuan Jia、Chan Li、Jianqi Xue、Jiabei Sun、Kaiyuan Wang、Yaling Gan、Jing Xu、Yaqin Shi、Xingjie Liang |
| 绘制单位 | College of Chemistry & Environmental Science, Key Laboratory of Medicinal Chemistry and Molecular Diagnosis of the Ministry of Education, Hebei University、National Institutes for Food and Drug Control, No. 5, Huatuo Rd., Daxing District、Laboratory of Cont |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 1–Results of irinotecan hydrochloride (nano) micelle interference test.”的人还看了
-
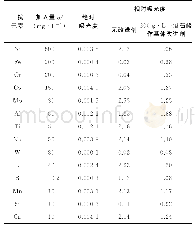
- 表2 凝胶法干扰实验预试验结果 (湛江博康海洋, n=2) Tab.2 Pre-test results of gel interference test (Zhanjiang Bokang Marine Biological Co., L
-
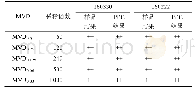
- 表3 凝胶法干扰试验预试验结果 (福州新北生化, n=2) Tab.3 Pre-test results of gel interference test (Fuzhou Xinbei Biochemical Industry Co., L