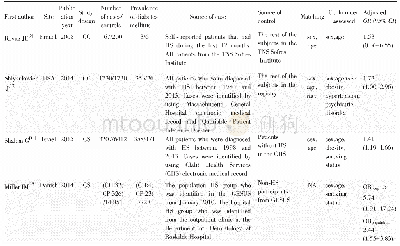
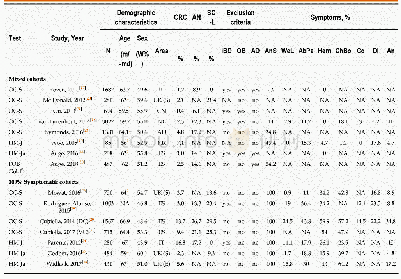
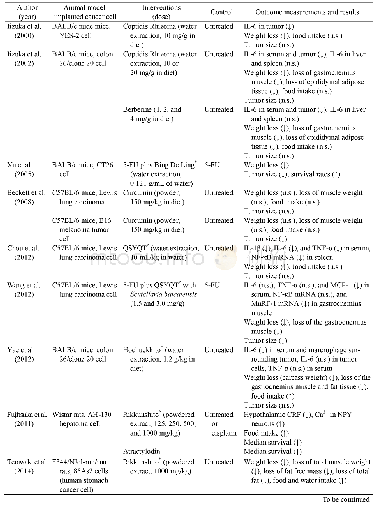
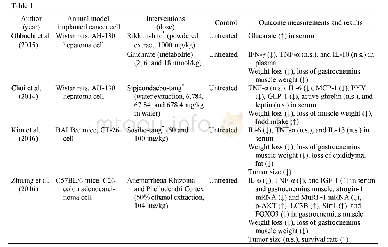
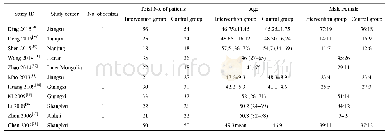
《Table 1.Study characteristics for the included studies.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《华蟾素注射液治疗中晚期原发性肝癌的统评价和Meta分析(英文)》
A total of 233 studies were identified through electronic searches of CENTRAL in The Cochrane Library(n=1),Pub Med(n=65),EMBASE(n=24),and four Chinese Databases(CBM,VIP,CNKI,Wangfang)(n=1089) .Figure 1 demonstrates the details of the study selection and search process.A total of 732 publications were initially retrieved,from which 24 RCTs concerning cinobufacini injection in liver cancer were identified.Moreover,13 trials were subsequently excluded for the following reasons:1) one was excluded as it was a meta-analysis[21];2) three studies evaluated the role of the cinobufacini tablet,not the injection;3) four studies reported incomplete and incomprehensive outcomes and did not meet the inclusion criteria;and 4) five studies were not randomly assigned.Overall,11 RCTs consisting of 728 patients,including 370 patients who were treated with cinobufacini injection met the inclusion criteria and were analyzed in this study[22–32].Table 1 shows the study characteristics for the included trials.Table 2 displays the methodological quality of these trials.Figure 2 demonstrates the low potential for publication bias.
| 图表编号 | XD0045070400 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.04.01 |
| 作者 | 董志勇、邱仙土、Stacy A.Kujawa、S.M.Shariful Islam、张杰 |
| 绘制单位 | 福建莆田学院附属医院普通外科、福建莆田学院附属医院普通外科、美国西北大学Feinberg医学院Robert H.Lurie医学研究中心、澳大利亚墨尔本迪肯大学健康学院、中国人民解放军联勤保障部队第九八八医院中医科 |
| 更多格式 | 高清、无水印(增值服务) |