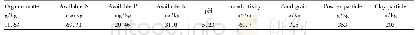《Table 2 Basic properties of QA-OMPFEKs and QA-TMPFEK-40 at room temperature》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《基于乌尔曼偶联反应制备的密集季铵化型全钒液流电池阴离子交换膜(英文)》
a) Samples with the counter-ion of Br-;b) samples with the counter-ion of SO42-.
By varying the loading amount of octa-benzylmethylcontaining mononer b,four QA-OMPFEKs with IECs ranging from 0.96 to 2.05 mmol g-1were obtained,as shown in Table 2.At room temperature,the water uptake and swelling ratio of QA-OMPFEKs increase monotonically with the increasing IEC.For example,the water uptake of QA-OMPFEK-10 with the counter-ion of Br-is9.9%,while that of QA-OMPFEK-25 is 18.2%.The Brconductivity of QA-OMPFEKs also increases with the increasing IEC,from 1.24 mS cm-1of QA-OMPFEK-10to 4.18 mS cm-1of QA-OMPFEK-25.This increase in Brconductivity can be mainly attributed to the higher ionic content and higher degree of hydrophilicity of the QA-OMPFEKs with higher IECs.Since sulfate is the main charge carrier of the AEM in the VRFBs with sulfuric acid-based electrolyte[3],the water uptake,swelling ratio and conductivity of the SO42-form AEMs were also characterized and listed in Table 2.The SO42-conductivity is found to be higher than the Br-conductivity,which may be attributed to the higher mobility of SO42-and higher water uptake of samples in SO42-form compared to Br-form[41].Weiber et al.[42]reported a series of polysulfone based AEMs with highly localized cation groups.The QA sample c4PAES-1.8Q had an IEC of1.8 mmol g-1,a water uptake of 28%,and a Br-conductivity of 1.3 mS cm-1at 20°C.Yun et al.[43]reported a QA functionalized poly(aryl ether ketone)s with an IEC of 1.54 mmol g-1which showed a water uptake of 36%and a SO42-conductivity of 5.6 mS cm-1at 30°C.Therefore,the anion conductivity of QA-OMPFEK-20 is comparable to these similar systems with densely localized ionic groups.
| 图表编号 | XD0039959900 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.01 |
| 作者 | 陈煜、林起浪、郑玉婴、于岩、陈栋阳 |
| 绘制单位 | College of Materials Science and Engineering, Fuzhou University、College of Materials Science and Engineering, Fuzhou University、College of Materials Science and Engineering, Fuzhou University、College of Materials Science and Engineering, Fuzhou University |
| 更多格式 | 高清、无水印(增值服务) |