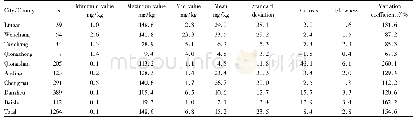
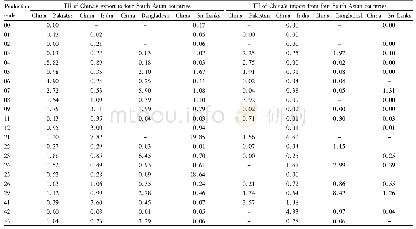
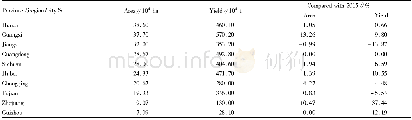
《Table 4–Cl distribution in the products of PVC pyrolysis (Ma et al., 2002) .》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Review on fate of chlorine during thermal processing of solid wastes》
The releasing behavior of HCl is influenced by experimental conditions,catalysts/absorbents and interactions between volatiles and residues(McNeill et al.,1995;Miranda et al.,1999b;Ma et al.,2002;Wikstr?m et al.,2003a;López et al.,2011,2012;Zhou et al.,2016).Table 4 shows the Cl distribution in the products produced from the pyrolysis of PVC(Ma et al.,2002).More than 90%of total Cl in PVC is released as HCl in the first stage(200–360°C).A lower operating pressure like vacuum and a longer retention time at operating temperatures may enhance the release of HCl(Miranda et al.,1999b,2001;Ma et al.,2002).The presence of oxygen(O2)in the atmosphere will lead to the formation of Cl2from HCl through the Deacon reactions(Wikstr?m et al.,2003a,2003b).The addition of catalysts/absorbents(e.g.,Fe2O3and CaO)can significantly fix the HCl and change the distribution of Cl in products,as it is discussed in detail in the following part of this review.
| 图表编号 | XD0033523100 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.04.15 |
| 作者 | Peng Lu、Qunxing Huang、A.C.(Thanos) Bourtsalas、Nickolas J.Themelis、Yong Chi、Jianhua Yan |
| 绘制单位 | State Key Laboratory of Clean Energy Utilization,Zhejiang University、State Key Laboratory of Clean Energy Utilization,Zhejiang University、Earth Engineering Center,Columbia University、Earth Engineering Center,Columbia University、State Key Laboratory of Cle |
| 更多格式 | 高清、无水印(增值服务) |