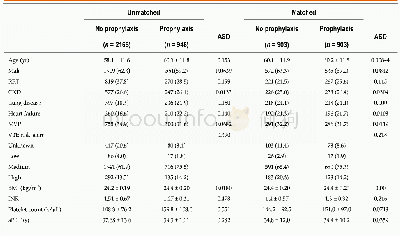
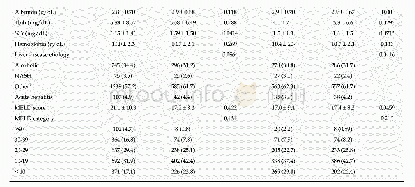
《Table 3 Differences in baseline characteristics between chemotherapy,chemoradiation therapy and che
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
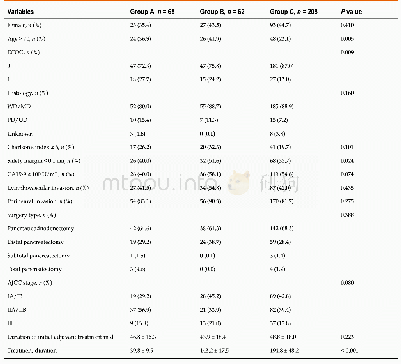
《Comparison of efficacy between adjuvant chemotherapy and chemoradiation therapy for pancreatic cancer: AJCC stage-based approach》
The limitations of this study are as follows.First,there can be an inherent selection bias of a single-center retrospective study design.Due to the nature of the study,the baseline characteristics of the groups were different.Compared with other groups,the CRT-SCT group had a higher proportion of young patients and Eastern Cooperative Oncology Group performance status of zero with a lower proportion of patients with a free surgical margin≤1 mm.Patients with better performance status might have been selected for CRT-SCT,which could potentially bias the results in favor of CRT-SCT.Furthermore,it is now well known that radiation therapy increases the local recurrence-free survival in patients with a surgical margin≤1 mm.However,the proportion of these patients were highest in the SCT group,which may be a major selection bias.Second,the statistical power can be weak because the total number of patients was relatively small in this study.Moreover,because the incidence of tumor recurrence and death was few in stage I,we were statistically unable to further evaluate OS and RFS in stages I and II separately.Third,this study excluded patients treated with adjuvant chemotherapy with regimens other than FL or gemcitabine.Recently,modified FOLFIRINOX showed its superiority compared to gemcitabine alone in the adjuvant settings and is a preferred in fit patients.However,the use of modified FOLFIRINOX as an adjuvant treatment is limited in Korea because it has not been approved for reimbursement by the Korean healthcare system.Because this study is limited by a small number of patients and retrospective design,a welldesigned clinical trial that prospectively compares SCT and/or CRT should be followed.
| 图表编号 | XD00229179400 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2020.09.24 |
| 作者 | Min Su You、Ji Kon Ryu、Gunn Huh、Jung Won Chun、Woo Hyun Paik、Sang Hyub Lee、Yong-Tae Kim |
| 绘制单位 | Internal Medicine and Liver Research Institute, Seoul National University Hospital、Internal Medicine and Liver Research Institute, Seoul National University Hospital、Internal Medicine and Liver Research Institute, Seoul National University Hospital、Intern |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 3 Differences in baseline characteristics between chemotherapy,chemoradiation therapy and chemotherapy plus chemor”的人还看了
-
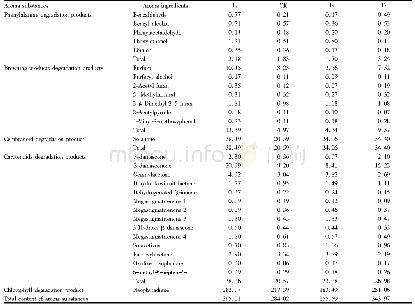
- Table 3 Effects of different temperature differences between dry and wet bulbs in yellowing stage on content of aroma su
-
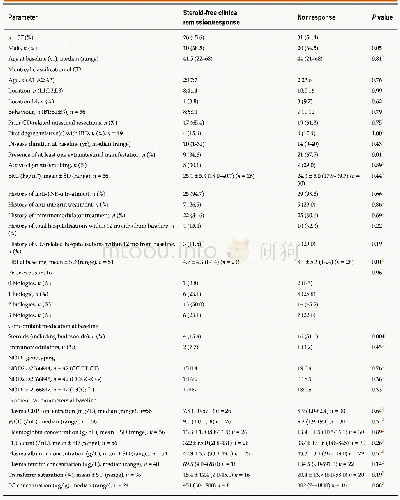
- Table 3 Comparison of baseline characteristics between the subgroups of patients with steroid-free clinical response ver
-
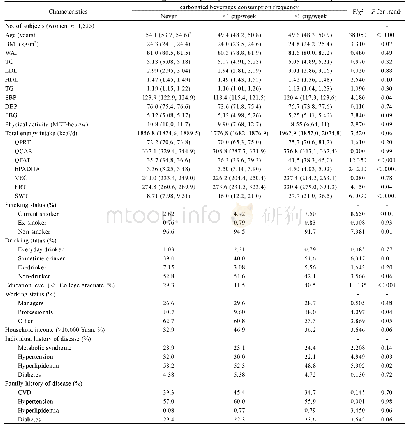
- Table 2 Baseline characteristics according to the frequency of carbonated beverages consumption in female
-
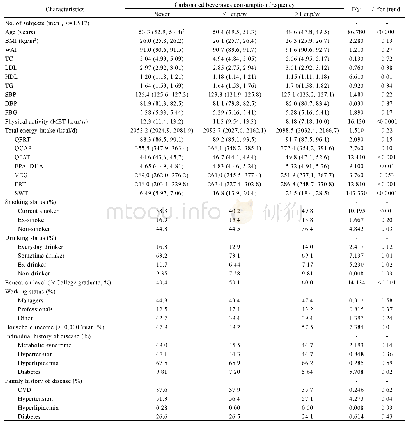
- Table 1 Baseline characteristics according to the frequency of carbonated beverages consumption in male