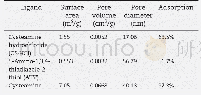
《Table 1 Effect of catalyst on the acylation of 2-acetylfuran》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《From Furan to High Quality Bio-based Poly(ethylene furandicarboxylate)》
a Molar ratio of catalyst to 2-acetylfuran=1:1;b Conversion=1-(remained2-acetylfuran/charged 2-acetylfuran);c Maximum yield of 2,5-DAF of the reaction;d Selectivity=Yield/Conversion
For the synthesis of 2,5-DAF,2-acetylfuran obtained above was further acylated by acetic anhydride via Friedel-Crafts reaction.In order to restrain the ring opening reaction of2-acetylfuran,relativelyweakacids,suchas p-toluenesulfonic acid,phosphoric acid or methanesulfonic acid,were used as the catalyst.Figure 2 shows the yields of2,5-DAF as a function of reaction time catalyzed by different catalysts.Obviously,a maximum yield of 2,5-DAF along with reaction time was observed no matter which catalyst was used.The conversion of 2-acetylfuran,the maximum yield of2,5-DAF and selectivity of this reaction using different catalysts are listed in Table 1.Undoutedly,methanesulfonic acid was the optimal catalyst for the reaction.It is known that2,5-DAF is one of the most important furan derivatives and several methods have been tried for its preparation.However,the harsh reaction conditions,including ultra low temperature(-78°C)and dangerous catalysts(such as n-Bu Li)are necessary[35].Up to now,literature information is hardly available about the synthesis of 2,5-DAF under mild conditions.In this work,the Friedel-Crafts reaction catalyzedby methanesulfonic acid was conducted at 120°C and2,5-DAF was synthesized successfully,though the yield was only about 40.2%.
| 图表编号 | XD0020170500 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.06.01 |
| 作者 | Jing-Gang Wang、Xiao-Qing Liu、Jin Zhu |
| 绘制单位 | Ningbo Institute of Materials Technology and Engineering,Chinese Academy of Sciences、University of Chinese Academy of Sciences、Key Laboratory of Bio-based Polymeric Materials of Zhejiang Province、Ningbo Institute of Materials Technology and Engineering,Ch |
| 更多格式 | 高清、无水印(增值服务) |