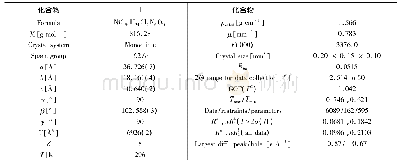
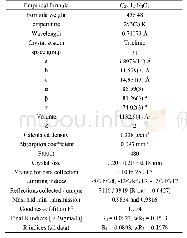
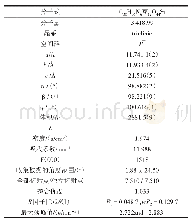
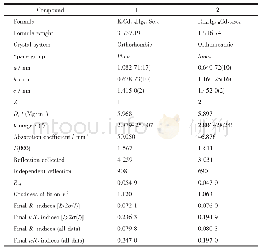
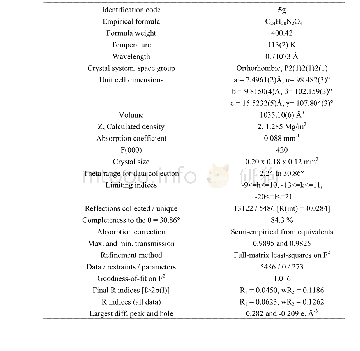
《表S2化合物5g晶体结构数据》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"基于α-溴代酮的催化不对称合成3,3-螺环丙基吲哚酮和乙烯基环丙烷(英文)"》
Given the biological importance of 3,3-spirocyclopropyl oxindoles,[13]synthesis of chiral spirocyclopropyl oxindoles has been intensively investigated,particularly in the recent decade,and a series of metal-mediated and organocatalytic asymmetric cyclopropanations have been accordingly developed from specific oxindole derivatives,providing facile assemblies of such chiral molecular motifs.[5c~6f,6a~6h,7a~7c]Considering the easy availability of cinchona alkaloids and their derivatives as chiral catalysts,and the robustness of the Gaunt protocol in synthesis of chiral cyclopropanes,we were interested in exploring new synthesis of chiral 3,3-spirocyclopropyl oxindoles via catalytic ammonium ylide-mediated asymmetric cyclopropanation of 3-(substituted methylene)oxindoles.To test the feasibility of the suspected reaction,the racemic cyclopropanation between(E)-1-benzyl-3-(benzoylmethylene)oxindole(2a)and a quaternary ammonium salt prepared fromα-bromoacetophenone(1a)and 1,4-diazabicyclo[2.2.2]-octane(DABCO)was first examined.Thus,the ammonium salt(0.15 mmol)was treated with 2a(0.10 mmol)and Na2CO3(0.15 mmol)in 1,4-dioxane with stirring at 90℃for 3 h,delivering the expected spirocyclopropyl oxindole rac-3a in 70%isolated yield with>20∶1 dr diastereoselectivity(Eq.1).
| 图表编号 | XD00140731700 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2020.01.01 |
| 作者 | 罗京华、耿玮笙、曹仕轩、贺峥杰 |
| 绘制单位 | 南开大学化学学院元素有机化学国家重点实验室、南开大学化学学院元素有机化学国家重点实验室、南开大学化学学院元素有机化学国家重点实验室、南开大学化学学院元素有机化学国家重点实验室、天津化学化工协同创新中心 |
| 更多格式 | 高清、无水印(增值服务) |