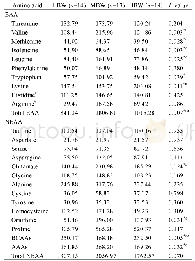
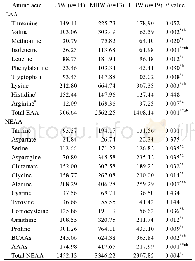
《Table 6 Energy difference among three hydroxyhexanoic acid adsorption models》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Environmentally-Friendly Catalytic Oxidation of Cyclohexanone with 30% H_2O_2 Solution: A Comparison Study between Hollow Titanium Silicate and Dealuminated HBEA Zeolites》
As shown in Table 5,the Lewis acid species in Al atoms do not have the activation effect on enhancing the nucleophilic attack capability of H2O2 molecules.Here,the adsorption models of 6-hydroxyhexanoic acid molecules on the trigonal Al sites are calculated,as illustrated in Figure 5.There are three O atoms in one6-hydroxyhexanoic acid molecule,which can possibly be absorbed by trigonal Al species via the acceptor-donor interaction between Al sites of active sites and these O atoms.It is found that the Al…O bond distance in model(b)is shorter than that of other two models(a)and(c),which suggests that model(b)is the most possible one for further oxidation reaction.Table 6 gives the corresponding adsorption energy for these three models.When the adsorption energy of model(b)is defined as 0,then that of models(a)and(c)is 74.46 kJ/mol and 32.23kJ/mol,respectively.Therefore,we can infer that the O atom of carbonyl group is apt to be adsorbed on trigonal Al sites.And in model(b),the charge of C atom of terminal C-OH group is-0.180,which is smaller than that in the original molecule(0.054).It is indicated that the terminal C-OH groups cannot be activated to accept the nucleophilic attack by H2O2 molecules.Thus,according to the above-mentioned results,the reason why few adipic acid produced in DHBEA zeolite catalyzed system can be well explicated.
| 图表编号 | XD0011307800 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.12.30 |
| 作者 | Xia Changjiu、Zhao Yi、Zhu Bin、Lin Min、Peng Xinxin、Dai Zhenyu、Shu Xingtian |
| 绘制单位 | State Key Laboratory of Catalytic Materials and Reaction Engineering, Research Institute of Petroleum Processing SINOPEC、State Key Laboratory of Catalytic Materials and Reaction Engineering, Research Institute of Petroleum Processing SINOPEC、State Key Lab |
| 更多格式 | 高清、无水印(增值服务) |