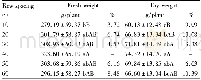
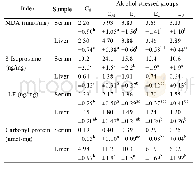
《Table 4Effects of TOPE on lipid profile and renal function in RIF-INH-induced hepatotoxicity in Wis
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Telfairia occidentalis (Cucurbitaceae) pulp extract mitigates rifampicinisoniazid-induced hepatotoxicity in an in vivo rat model of oxidative stress》
Group 1:negative control,receiving 10 ml/kg body weight(b.w.)distilled water;Group 2:disease control group,receiving RIF+INH(both 100 mg/kg b.w.,the same below)+10 mL/kg distilled water;Group 3:receiving RIF-INH+TOPE(125 mg/kg b.w.);Group 4:receiv
The effects of experimental treatments on body weights are presented in Table 1.The treatments produce significant alterations in the weights(P<0.0001).The study found that statisticallysignificant differences in body weight were present between all treated groups and the disease control group.TOPE(125–500 mg/kg),the reference drug silymarin(50 mg/kg)and100 mg/kg RIF+100 mg/kg INH demonstrated a minimal marked effect on weights of experimental animals(Table 1).At the 8th week,animals in Group 3,receiving TOPE 125 mg/kg,had significantly elevated body weights compared to their initial body weights.The reference drug(Group 6)and the middle dose of TOPE(250 mg/kg;Group 7)significantly(P<0.05 and P<0.01,respectively)increased body weights from the first week to the eighth week compared to the initial.Treatment with RIF-INH produced a marked reduction in liver weights compared to the negative control.Significant(P<0.001)increase in liver weight occurred in rats given TOPE 250 mg/kg plus RIF-INH 100 mg/kg compared to the disease control group(Table 1).Other experimental doses,the reference and normal control did not exhibit any marked effect on liver weights.
| 图表编号 | XD0044093000 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.01 |
| 作者 | Lucky Legbosi Nwidu、Yibala Ibor Oboma |
| 绘制单位 | Experimental Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, University of Port Harcourt、Medical Laboratory Sciences, Faculty of Basic Medical Sciences, College of Health Sciences, Niger Delta University |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 4Effects of TOPE on lipid profile and renal function in RIF-INH-induced hepatotoxicity in Wistar albino rats.”的人还看了
-

- Table 4Effect of force-feeding of C.dichotoma extract on lipid profile in rats fed high-fat diet after 4 weeks.
-
- Table 4Effects of different nitrogen sources on colony diameter and spore production of Colletotrichum horii