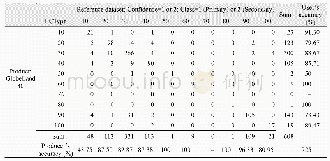
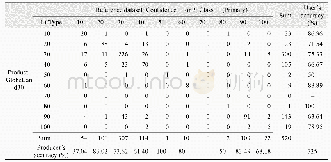
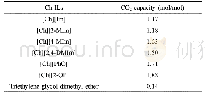
《Table 1 CO2absorption capacity of the Ch-ILs at 30°C and atmospheric pressure》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Choline-based ionic liquids for CO_2 capture and conversion》
The resultant Ch-ILs were applied in the CO2capture,and their CO2absorption capacities are shown in Table 1.Obviously,each Ch-IL has CO2absorption capacity higher than1.0 mol per mol IL,suggesting the strong interaction between the IL and CO2,which probably originated from the chemical absorption by the IL.In contrast,the CO2capacity of each Ch-IL was significantly greater than that of triethylene glycol dimethyl ether(0.14 mol/mol).To explore the influences of temperature and pressure on the CO2capacities,the CO2adsorption capacities of these ILs were examined in the temperature range of 30–60°C at 1 atm,and in the pressure range of 0.3–1 atm at 30°C,respectively,as shown in Tables S1 and S2(Supporting Information online).It is obvious that both temperature and pressure have a little influence on the CO2adsorption capacity,deduced from the facts that the CO2absorption capacity decreased slightly with the temperature,and increased slightly with the pressure under the experimental conditions.
| 图表编号 | XD0034633400 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.01 |
| 作者 | Ruipeng Li、Yanfei Zhao、Zhiyong Li、Yunyan Wu、Jianji Wang、Zhimin Liu |
| 绘制单位 | Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering,Henan Normal University、Beijing National Laboratory for Molecular Sciences, Key Laboratory of Colloid, Interface and Thermodynamics, |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 1 CO2absorption capacity of the Ch-ILs at 30°C and atmospheric pressure”的人还看了
-

- 表2 葫芦河径流量和降水量 (30 a尺度) 丰枯变化周期统计表Table 2The varying cycle at the scale of 30 years of runoff and precipitation in the Hul