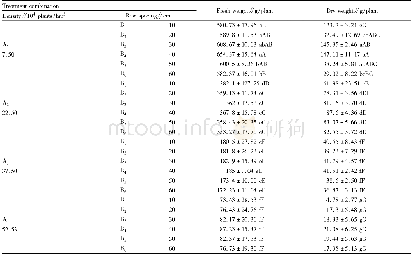
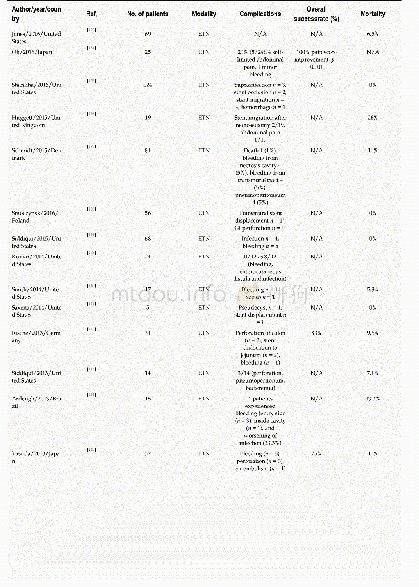
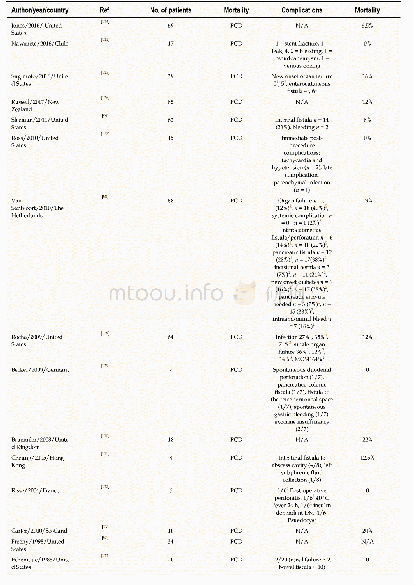
《Table 5–Literature summary about the effects of chlorides on the evaporation of heavy metals.》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Review on fate of chlorine during thermal processing of solid wastes》
Previous research(Table 5)reveals that the increase of Cl content from either organic chlorides(e.g.,PVC)or inorganic chlorides(e.g.,NaCl,KCl,CaCl2and FeCl3)will increase the amount of most of heavy metals(e.g.,Cd,Zn,Cr,Cu)partitioned in the fly ash by the formation of easily evaporated chlorides.These heavy metal chlorides,which can be formed through direct chlorination and indirect chlorination mechanisms,have higher vapor pressures than corresponding oxides.For indirect chlorination,chlorides firstly form HCl and/or Cl2by reacting with H2O and/or O2(Eqs. (8)–(9)) .The generated HCl or Cl2then react with heavy metals or heavy metal oxides to form chlorides(Eqs. (6)–(7)) .The direct chlorination converts the heavy metal oxides(MO)into chlorides(MCl2)by Eq.(12)(Jakob et al.,1996) .
| 图表编号 | XD0033523300 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.04.15 |
| 作者 | Peng Lu、Qunxing Huang、A.C.(Thanos) Bourtsalas、Nickolas J.Themelis、Yong Chi、Jianhua Yan |
| 绘制单位 | State Key Laboratory of Clean Energy Utilization,Zhejiang University、State Key Laboratory of Clean Energy Utilization,Zhejiang University、Earth Engineering Center,Columbia University、Earth Engineering Center,Columbia University、State Key Laboratory of Cle |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 5–Literature summary about the effects of chlorides on the evaporation of heavy metals.”的人还看了
-

- Table 2 Effects of the two silicon fertilizers on chlorophyll content and yield of maize and T.truncates
-

- Table 5 Comparison of the effects on the CPU time of the different methods to speed up the transformation.Ratio of the t
-
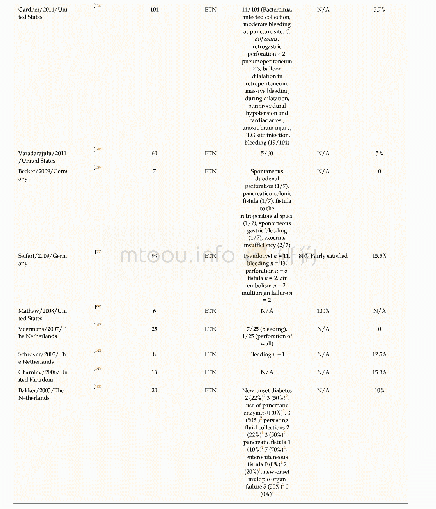
- Table 2 Peer-reviewed literature reports on the clinical outcomes of patients undergoing endoscopic transluminal necrose