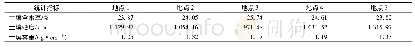《Table 2 Physical structural properties of the samples》
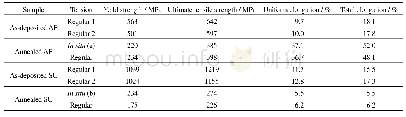
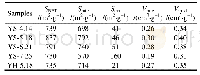
Figure 7(a)and(b)are 29Si MAS NMR spectra of sample YS-5.18 and the corresponding dried gel YP-5.18,respectively.The resonances atδ=-108~-105 are assigned to Si(4Si,0Al)sites,and thoseδ=-105~-95 are assigned to Si(3Si,1Al)sites;moreover,those atδ=-95~-92,-92~-86,and-86~-80,are respectively assigned to the Si(2Si,2Al),Si(1Si,3Al),and Si(0Si,4Al)[26].The calculated framework Si/Al ratio of sample YS-5.18 is about 1.65(Table 1),which is slightly lower than 1.73 detected by EDS.It can be seen from Fig.7(b)that the resonances assigned to Si(0Si,4Al),Si(1Si,3Al)and Si(2Si,2Al)sites can be obviously observed in dried gel YP-5.18,indicating that the primary zeolite framework has been practically formed in dried gel yielded from the gel mixture after treated at 60℃for 24h.The calculated framework Si/Al ratio of dried gel YP-5.18 is about 1.27(Table 1),which is rather lower than 1.65 of the final product YS-5.18.The aforementioned results suggest that the primary zeolite framework formed during the preparation of the dried gel has a rather lower Si/Al ratio as compared with the one in final products.The calculated framework Si/Al ratio and the different aluminium coordination are shown in Table 1.It can be inferred that the primary zeolite framework formed during preparing dried gel is rich in Al-,and Si-species entering the zeolite framework fall behind the Al-species.Combining with the FT-IR result as shown in Fig.5,it can be further deduced that some building units or minicrystals with primary zeolite framework,which are not yet detected by XRD or TEM because of very small spatial scale,have been really formed during the preparation of the dried gel precursors.
| 图表编号 | XD0043167400 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.02.01 |
| 作者 | 杜艳泽、杨晓娜、宁伟巍、孔庆岚、秦波、郑家军、李文林、李瑞丰 |
| 绘制单位 | 太原理工大学能源化工与催化研究中心煤科学与技术教育部与山西省重点实验室、中国石油化工集团大连石油化工研究院、太原理工大学能源化工与催化研究中心煤科学与技术教育部与山西省重点实验室、太原理工大学能源化工与催化研究中心煤科学与技术教育部与山西省重点实验室、太原理工大学能源化工与催化研究中心煤科学与技术教育部与山西省重点实验室、中国石油化工集团大连石油化工研究院、太原理工大学能源化工与催化研究中心煤科学与技术教育部与山西省重点实验室、太原理工大学能源化工与催化研究中心煤科学与技术教育部与山西省重点实验室、太原理 |
| 更多格式 | 高清、无水印(增值服务) |
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式