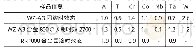
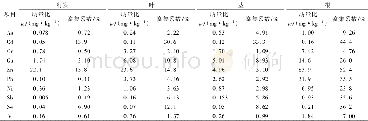
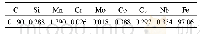《Table 3.First-order interaction coefficients of elements in liq-uid steel at 1873 K[18]》
![《Table 3.First-order interaction coefficients of elements in liq-uid steel at 1873 K[18]》](http://bookimg.mtoou.info/tubiao/gif/BJKY201803004_12800.gif) 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Novel mechanism for the modification of Al_2O_3-based inclusions in ultra-low carbon Al-killed steel considering the effects of magnesium and calcium》
where R and T are the ideal gas constant and Kelvin temperature,respectively;a[i](i=Al,Ca or Mg)represents the activity of element i in liquid steel relative to dilute solution,and ak(k=Al2O3,Ca O or Mg O)denotes the activity of components in inclusions relative to pure solid state.a[i]is calculated by Eq.(7),and the activity coefficient of each element in liquid steel is calculated using Eq.(8),where jie is the first-order interaction coefficient of elements j to i relative to dilute solution.The values of these coefficients are presented in Table 3.The calculated activity coefficient of each element in liquid Type A steel is listed in Table 4.Some academic papers[4,12]have reported that second-order interaction coefficients between Ca and O,between Al and O,and between Mg and O also influence their activity.In the present study,only first-order interaction coefficients were taken into account given that,similar to the method used by Jiang et al.[3],since the steel grades used in the present study contain low amounts of alloy element components.Moreover,our previous work has already confirmed present method could obtain a reasonable result.
| 图表编号 | XD002863100 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.03.01 |
| 作者 | Jing Guo、Shu-sen Cheng、Han-jie Guo、Ya-guang Mei |
| 绘制单位 | School of Metallurgical and Ecological Engineering, University of Science and Technology Beijing、Beijing Key Laboratory of Special Melting and Reparation of High-end Metal Materials, University of Science and Technology Beijing、School of Metallurgical and |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 3.First-order interaction coefficients of elements in liq-uid steel at 1873 K[18]”的人还看了
-

- Table 3 Leaching results of clinker in alkaline solution at 383 K and Na OH concentration of 160 g/L
-

- Table 2.Diffusion coefficients at 1300°C of elements in the present alloy and those reported for other Co-based alloy sy