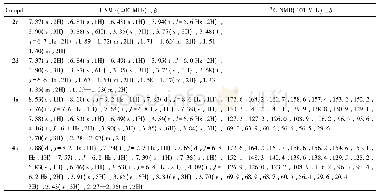
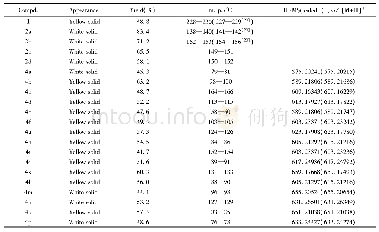
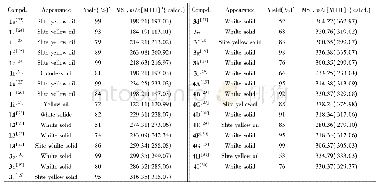
《Table 3 Kine tic data of N2O de composition ove r 0.03Y-Co3O4and K-modifie d catalysts unde r vario
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《一步水热合成Y-Co_3O_4复合氧化物催化剂及催化分解N_2O(英文)》
Under the reaction conditions of 1%N2O+2%O2+Ar,1%N2O+2%O2+8.2%H2O+Ar,nitrous oxide can be completely decomposed at 350 and 375℃on K-supported catalyst,respectively.In the reactant gases of non-oxygen,oxygen-alone,and oxygensteam together,the catalyst activity decreases in turn,both apparent activation energy(Ea)and preexponential factor(A)increase(listed in Table 3).Evidently,there is a dynamic compensation effect betw een ln A and Eaw ith ln A=6.62787+0.12828Ea for 0.03Y-Co3O4,and ln A=5.21743+0.16299Eafor0.02K/0.03Y-Co3O4catalyst.
| 图表编号 | XD0039694900 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.04.01 |
| 作者 | 赵天琪、高强、李和健、徐秀峰 |
| 绘制单位 | 烟台大学化学化工学院应用催化研究所、烟台大学化学化工学院应用催化研究所、烟台大学化学化工学院应用催化研究所、烟台大学化学化工学院应用催化研究所 |
| 更多格式 | 高清、无水印(增值服务) |