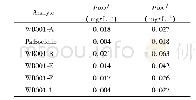
《Table 2Maximum residues limits (MRLs) of mycotoxins》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《"Extrinsic harmful residues in Chinese herbal medicines: types,detection, and safety evaluation"》
EC,European Commission Regulation;EP,European Pharmacopoeia 9th;BP,British Pharmacopoeia(2017);Ch P,Chinese Pharmacopoeia(2015);JP,Japanese Pharmacopoeia 17th;USP,United States Pharmacopeia 40th;GSMPP,Green standards of medicinal plants and prep
It is difficult to detect mycotoxins in the complex matrix of CHMs because the MRLs of mycotoxins are very low.The matrices of CHMs are complex,so there are some limitations that the sample testing process and detection methods in agricultural products are directly applied.Nowadays a variety of purification methods have been developed for the complex matrix of CHMs,including solid-phase extraction(SPE),liquid-liquid extraction,chemical adsorption,“quick,easy,cheap,effective,rugged and safe”(Qu ECh ERS),and immunoaffinity column(IAC)(Kong et al.,2014;Wen,Kong,Hu,Wang,&Yang,2014;Zhang et al.,2017) .Table 3 showed the mycotoxin pollution status of CHMs that may provide a reference for the safety of CHMs on the early warning of mycotoxins contamination and reduce the risk of mycotoxins in clinic.
| 图表编号 | XD007989600 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2018.04.18 |
| 作者 | Cong-min Liu、Jia-an Qin、Xiao-wen Dou、Mei-hua Yang、Xiao-bo Sun |
| 绘制单位 | Key Laboratory of Bioactive Substances and Resources Utilization of Chinese Herbal Medicines, Ministry of Education, Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences, Peking Union Medical College、Key Laboratory of Bioactive Su |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 2Maximum residues limits (MRLs) of mycotoxins”的人还看了
-

- Table 6 Estimated Ccontent of shoot residue, root residue, organic manure and soil Cinput, and seed coton yield as afect