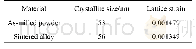《Table 1–Reactant concentrations (n) , mean crystallite sizes and the ratios of the intensities of (
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Enhanced adsorption of arsenate by spinel zinc ferrite nano particles: Effect of zinc content and site occupation》
The ZnxFe3-xO4(x=0.2,0.4,0.6,0.8 and 1)samples were synthesized using a facile hydrothermal method13.Briefly,Fe(NO3)3·9H2O and Zn(NO3)2·6H2O were dissolved in 50 mL of C2H5OH,and 18 mmol CH3COONa was subsequently added into the mixture.The molar amounts of FeNO3·9H2O and Zn(NO3)2·6H2O are listed in Table 1.After vigorously stirring for about 5 min,the mixture was transferred into a Teflon-lined steel autoclave and thermally treated for 24 hr at 200°C.Then the mixture was naturally cooled down to room temperature The solid products of formula ZnxFe3-xO4were collected by centrifugation and washed with ethanol/deionized water five times.Then the products were dried at 50°C for 24 hr in a vacuum oven.
| 图表编号 | XD0052061100 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.05.15 |
| 作者 | Can Wu、Yunyun Xu、Si Xu、Jingwei Tu、Chen Tian、Zhang Lin |
| 绘制单位 | School of Environment and Energy, The Key Laboratory of Pollution Control and Ecosystem Restoration in Industry Clusters (Ministry of Education), South China University of Technology、Guangdong Engineering and Technology Research Center for Environmental N |
| 更多格式 | 高清、无水印(增值服务) |
查看“Table 1–Reactant concentrations (n) , mean crystallite sizes and the ratios of the intensities of (220) and (222) peaks”的人还看了
-

- Table 1–Effect of different concentrations of propranolol on zeta potential and particle size of the nanoparticles (mean
-

- Table 2Crystallite size,optical absorption parameters of BI-diisoQ film as a function of film thickness.
-
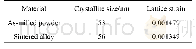
- Table 2 Variation in crystallite size and lattice strain of as-milled and sintered alloy obtained using Williamson-Hall