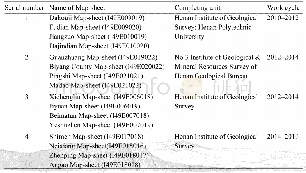
《Table 1 Examples of experimental features from somatic cell to iPSCs》
 提示:宽带有限、当前游客访问压缩模式
提示:宽带有限、当前游客访问压缩模式
本系列图表出处文件名:随高清版一同展现
《Differentiation of retinal ganglion cells from induced pluripotent stem cells: a review》
i PSCs:Induced pluripotent stem cells;VPA:Valproic acid.
Glaucoma is a common optic neuropathy and one of theleading causes of irreversible blindness in the world.It is characterized by the progressive degeneration of axons and the loss of retinal ganglion cells(RGCs).Approximately111.8 million people will be affected by glaucoma by 2040[1].The main risk factor for glaucomatous optic neuropathy is high intraocular pressure(IOP).Current glaucoma treatments that primarily target increased IOP,including topical eyedrops,laser treatment,and surgeries,but only slow the progression of RGC loss[2].There are an increasing number of recent reports on the development of neuroprotective therapies for glaucoma,that could be used as adjunctive treatments to lower IOP[3].These studies investigated neuroprotective strategies,including the delivery of a neurotrophic factor,the molecular application of anti-apoptosis and anti-inflammation treatments,and the reduction of oxidative stress[4].However,the clinical application is partially limited,because long-term maintenance of the supplementation is difficult and there is a possibility of compulsory repeated interventions.Recently,cell-based therapy has been demonstrated to be effective for the treatment of several diseases[5-8].The cells used for these therapies include mesenchymal stem cells(MSCs),dental pulp stem cells,embryonic stem cells(ESCs),and induced pluripotent stem cells(iPSCs)and so on.In 2006,Takahashi and Yamanaka[9]successfully reprogrammed mouse and adult fibroblasts to a pluripotent state by using four transcription factors(Oct3/4,Sox2,Klf4,and c-Myc)that are involved in the pluripotency maintenance in ESCs.The resulting cells,iPSCs,could form colonies that are morphologically similar to ESCs and are capable of differentiating into all three germ layer cell lineages.Since iPSCs could be reprogrammed by the somatic cells of a patient,they could maintain the total unique genomic information of each individual.These patientderived iPSCs could serve as a perfect in vitro model for genetic disease studies and thus have a promising role in the development of personalized treatment.These factors have resulted in an explosion of studies that attempt to exploit the reprogramming of somatic cells into iPSCs,reporting various modified protocols designed to improve the reprogramming efficiency and facilitate clinical application.For example,instead of retroviruses,multiple studies have used plasmid[10-11],mi RNA[12],and protein[13]as transcript factors delivery vectors to prevent the risk of insertional mutagenesis of the host cells.Other reports indicate that the addition of small molecules,such as valproic acid(VPA)[14],AZA5-aza-cytidine(AZA)[15],butyrate[16],vitamin C[17],transforming growth factor-β(TGF-β)receptor inhibitor(A-83-01)[18-19],MEK inhibitor(PD325901)[18-19],GSK3βinhibitor(CHIR99021)[18-19],and ROCK inhibitor(HA-100)[18-19]could enhance reprogramming efficiency and even replace the use of certain transcription factors in iPSCs generation protocols.Table 1 shows several examples of the experimental features of protocols to transform somatic cells into iPSCs.Insight is required regarding how to induce i PSCs to differentiate into the specialized cell fate of interest.An increasing number of reports have indicated that iPSCs could be differentiated into RGCs,photoreceptors,and retinal pigment epithelium(RPE)under appropriate conditions[20-21].The current review provides a perspective on the key methods that led to the differentiation of RGCs,and divulged the problems that must be solved before the iPSCs-derived RGCs could fulfill its potential in medical applications,such as the mechanisms of pathology,screening treatment drugs,and development of cell-based and patient-specific therapies targeting glaucoma and other optic neuropathies.
| 图表编号 | XD0032751000 严禁用于非法目的 |
|---|---|
| 绘制时间 | 2019.01.18 |
| 作者 | Shang-Li Ji、Shi-Bo Tang |
| 绘制单位 | Aier Eye Institute、Aier School of Ophthalmology,Central South University |
| 更多格式 | 高清、无水印(增值服务) |